An ideal gas, initially at 30°C and 100 kPa, undergoes the following cyclic processes in a closed system:
What will be an ideal response?
(a) In mechanically reversible processes, it is first compressed adiabatically to 500 kPa, then cooled at a constant pressure of 500 kPa to 30°C, and finally expanded isothermally to its original state.
(b) The cycle traverses exactly the same changes of state, but each step is irreversible with an efficiency of 80% compared with the corresponding mechanically reversible process. Note: The initial step can no longer be adiabatic.
Calculate Q, W, ?U, and ?H for each step of the process and for the cycle. Take 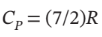 and
and 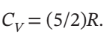
Is 303 K and 100 kPa, for which
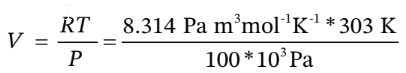
V = 0.0252 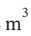 /mol. For adiabatic compression from 100 kPa to 500 kPa, we have
/mol. For adiabatic compression from 100 kPa to 500 kPa, we have
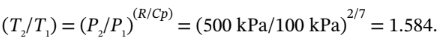 So, the temperature after compression is
So, the temperature after compression is
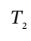 = 1.584*303 K = 480 K. The final volume after compression will then be
= 1.584*303 K = 480 K. The final volume after compression will then be
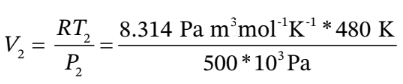
As always for an ideal gas, ?U = 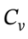 ?T = 2.5 R*(480 K – 303 K)) = 3679 J/mol, and
?T = 2.5 R*(480 K – 303 K)) = 3679 J/mol, and
?H = 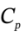 ?T = 3.5 R*(480 K – 303 K) = 5150 J/mol. For an adiabatic process, Q = 0, and W = ?U = 3679 J/mol.
?T = 3.5 R*(480 K – 303 K) = 5150 J/mol. For an adiabatic process, Q = 0, and W = ?U = 3679 J/mol.
If we cool the gas at constant pressure back to the original temperature, the enthalpy and internal energy go back to their original values, and we will have ?U = 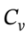 ?T = 2.5 R*(303 K – 480 K) = -3679 J/mol, and
?T = 2.5 R*(303 K – 480 K) = -3679 J/mol, and
?H = 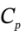 ?T = 3.5 R*(303 K – 480 K) = -5150 J/mol. During this process, the volume will decrease to R*303 K / 500000 Pa = 0.005039
?T = 3.5 R*(303 K – 480 K) = -5150 J/mol. During this process, the volume will decrease to R*303 K / 500000 Pa = 0.005039 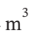 /mol. The work done will be
/mol. The work done will be
W = - P?V = -500000 Pa * (0.005039 – 0.007981) 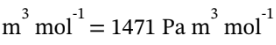 = 1471 J/mol. The heat removed can be found from
= 1471 J/mol. The heat removed can be found from
Q = ?U ?W = –3679 J/mol ? 1471 J/mol = –5150 J/mol.
Finally, if we expand the gas back to its original state isothermally, we will have ?U = ?H = 0 (since they only depend on temperature, which doesn’t change). For the isothermal process, we will have
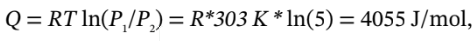 and W = -Q = -4055 J/mol.
and W = -Q = -4055 J/mol.
For the overall cycle, Q = 0 – 5150 + 4055 = -1095. W must then be 1095 for the cycle (so that the overall ?U is zero). To check, we can add up the three steps: W = 3679 + 1471 - 4055 = 1095. Of course ?U and ?H are zero for any cyclic process.
b. In the first step, if we compress it to 500 kPa with a process that is 80% efficient, and still adiabatic, then we will put in W = 3679/0.8 = 4599 J of work to do the compression. If Q is still zero, then we will have ?U = 4599 J = 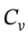 ?T, so ?T will be 221 K. In compressing the gas adiabiatically with an 80% efficient process, we will heat it to 524 K rather than 480 K. The enthalpy change will then be ?H =
?T, so ?T will be 221 K. In compressing the gas adiabiatically with an 80% efficient process, we will heat it to 524 K rather than 480 K. The enthalpy change will then be ?H = 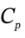 ?T = 3.5 R*(221 K) = 6431 J/mol. Because of the higher temperature, the molar volume will then be 0.008797
?T = 3.5 R*(221 K) = 6431 J/mol. Because of the higher temperature, the molar volume will then be 0.008797 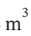 /mol rather than 0.007981
/mol rather than 0.007981 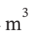 /mol that was found in (a).
/mol that was found in (a).
If we cool the gas back to the original temperature, as in the reversible case, the enthalpy and internal energy will go back to their original values, and we will have ?U =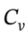 ?T = 2.5 R*(-221) = -4599 J/mol, and
?T = 2.5 R*(-221) = -4599 J/mol, and
?H =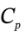 ?T = 3.5 R*(-221) = -6431 J/mol. The volume after this step will be the same as in part (a): R*303 K / 500000 Pa = 0.005039
?T = 3.5 R*(-221) = -6431 J/mol. The volume after this step will be the same as in part (a): R*303 K / 500000 Pa = 0.005039 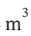 /mol. The reversible work required to cool the gas isobarically is then
/mol. The reversible work required to cool the gas isobarically is then
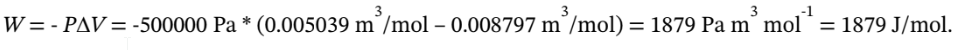 So, if our process is 80% efficient, the total work required will be 1879/0.8 = 2349 J/mol.
So, if our process is 80% efficient, the total work required will be 1879/0.8 = 2349 J/mol.
We still have ?U = -4599 J/mol = Q + W = Q + 2349, so Q = -6948 J/mol.
For the isothermal expansion, we will still have ?U = ?H = 0 (since they only depend on temperature, which doesn’t change). For a reversible isothermal process, we had 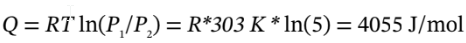 and
and
W = -Q = -4055 J/mol. If the process is only 80% efficient, then the work will be 0.8*(-4055) = -3244, and the heat requirement will be set by
Q = ?U ?W = 0 J/mol ? (3244 J/mol) = 3244 J/mol.
For the overall cycle with 80% efficiency in each step, Q = 0 – 6948 +3244 = -3704 J/mol. W must then be 3704 for the cycle (so that the overall ?U is zero). To check, we can add up the three steps:
W = 4599 + 2349 - 3244 = 3704 J/mol. Of course ?U and ?H are zero for any cyclic process, whether the steps are reversible or not. Note that the irreversibilities compound themselves so that the net work we put in here for the whole cycle is much more than 1.25 times (or 1/0.8) the net work that we put in for the reversible cycle in part (a).
You might also like to view...
A ____ inventory will make a prospective employer aware of the tools with which you are most familiar.
a. cognitive b. photo c. resume d. skills
The term MOST-Likely used to describe wheels that are closer together on one side of the vehicle than the other is:
A. toe change B. wheelbase difference C. thrust angle D. setback
The _____ is the only bright blue bird commonly found in the eastern half of the United States
a. blue jay b. eastern bluebird c. southern bluebird d. blue-winged robin
Cars with diesel engines have excessively high intake manifold vacuum.
Answer the following statement true (T) or false (F)