Solve the problem.The Van der Waals equation provides an approximate model for the behavior of real gases. The equation is P(V, T) = 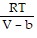 -
- 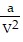 , where P is pressure, V is volume, T is Kelvin temperature, and a,b , and R are constants. Find the partial derivative of the function with respect to each variable.
, where P is pressure, V is volume, T is Kelvin temperature, and a,b , and R are constants. Find the partial derivative of the function with respect to each variable.
A. PV = 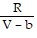 ; PT =
; PT = 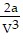 -
- 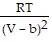
B. PV =  -
-  ; PT =
; PT = 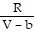
C. PV = - 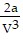 +
+  ; PT =
; PT = 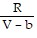
D. PV = 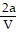 -
- 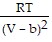 ; PT =
; PT = 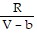
Answer: B
You might also like to view...
Classify the statement as true or false. If false, demonstrate why.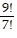 = 2!
= 2!
A. False; 72 ? 2 B. True
Use the Fundamental Counting Principle to solve the problem.A restaurant offers 7 entrees and 11 desserts. In how many ways can a person order a two-course meal?
A. 18 B. 77 C. 20 D. 154
Write the whole number in word form.35,203
A. three thousand five, two hundred three B. thirty-five hundred thousand, two hundred three C. thirty-five thousand, two hundred three D. thirty-five thousand, twenty-three
Solve using Cramer's rule, if possible. If it is not possible, state whether the system is inconsistent or has infinitely many solutions.
A. (5, 4) B. (-5, -4) C. (4, 5) D. (-4, 5)