Some data for pure compounds W and Z is given in the table below.

Mixtures of W and Z form an azeotrope at the following conditions: P=0.46 bar, T=50 ?C, and xW=0.86. At these conditions, the liquid mixture has a molar volume of V = 80.2 cm3/mol.
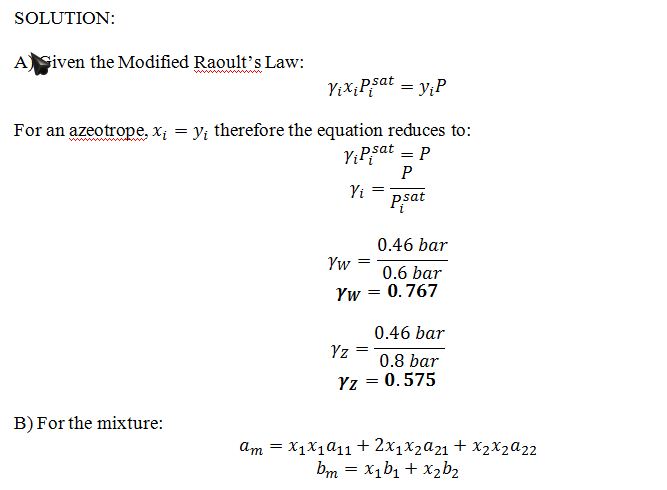
A) Determine the activity coefficients ?W and ?Z for the azeotropic mixture at 50 ?C.
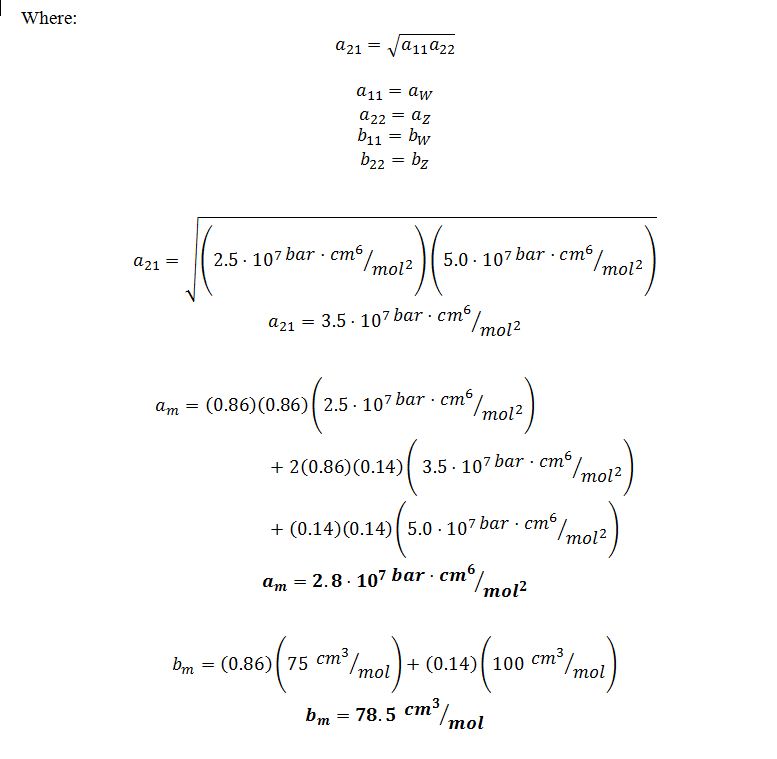
B) If you needed to model the azeotropic mixture using the van der Waals equation of state, what values of the parameters a and b would you use?
C) If you needed to model the azeotropic mixture at 50 ?C using the van Laar model, what values of the parameters L12 and L21 would you use?
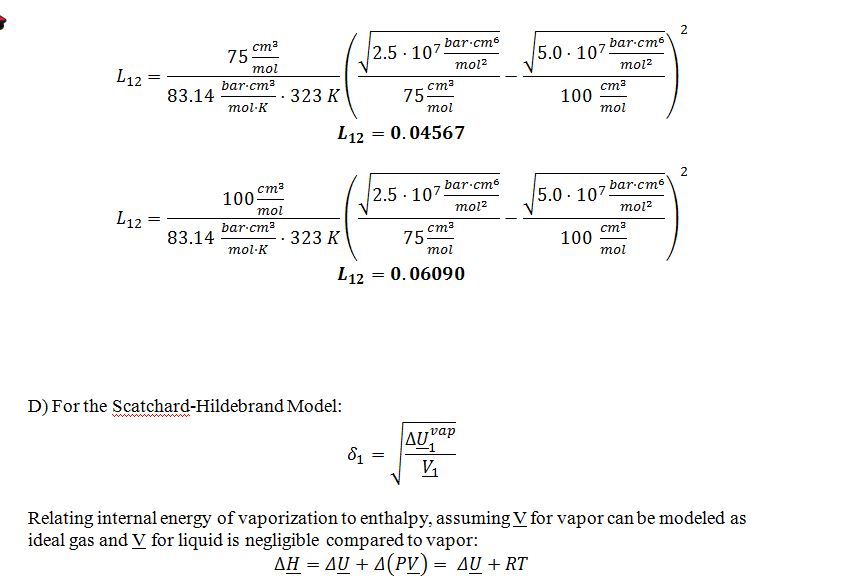
D) If you needed to model the azeotropic mixture at 50 ?C using the Scatchard Hildebrand model, what value would you use for the solubility parameter of compound W (?W)?
E) You are asked to determine whether mixtures of these compounds form an azeotrope at 30 ?C, and if so, the P and xWof that azeotrope.You have only the information in the table and the known 50 ?C azeotrope described above. Your teammate suggests trying two models: Wilson and Scatchard-Hildebrand. Give your evaluation of the potential use of each of these two models for this problem: is it impossible to apply without more information, is it possible to apply but not likely to be very accurate, or is it a reasonable model to use? Explain.
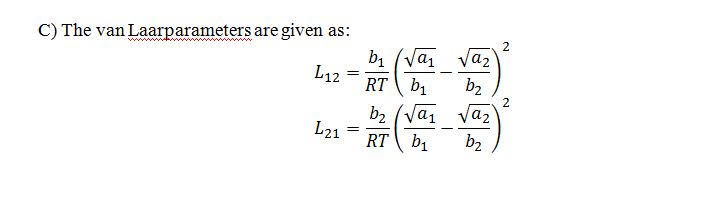

You might also like to view...
____ are used for recovery from disasters that threaten on-site backups.
A. Data archives B. Cloud storage sites C. Data backups D. Electronic vaulting sites
Round 0.56175 to three decimal places.
A. 0.560 B. 0.561 C. 0.562 D. 0.563
The size of many nails is determined by the penny system. This method determines the size of nails by their ____.
A. shank size B. length C. weight D. all the above
Condensation __________.
A. loses heat energy B. gains heat energy C. none of the above