Electron Speed: Given that the speed of an electron in the ground state of hydrogen is 2190 km/s, what is the speed of an electron in the n = 4 orbit of hydrogen?
A. 547 km/s
B. 31,000 km/s
C. 139 km/s
D. 178 km/s
E. 2180 km/s
Answer: A
You might also like to view...
A 15-mF capacitor and a 25-mF capacitor are connected in parallel, and charged to a potential difference of 60 V. How much energy is then stored in this capacitor combination?
a. 50 mJ b. 18 mJ c. 32 mJ d. 72 mJ e. 45 mJ
What is the wavelength of a photon having the same momentum as an electron that is traveling at 7.5 km/s?
(h = 6.626 × 10-34 J ? s, melectron = 9.11 × 10-31 kg)
Phobos and Deimos are probably captured comet nuclei
Indicate whether the statement is true or false
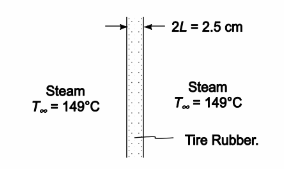
In the vulcanization of tires, the carcass is placed into a jig and steam at 149°C is admitted suddenly to both sides. If the tire thickness is 2.5 cm, the initial temperature is 21°C, the heat transfer coefficient between the tire and the steam is 150 W/(m2 K), and the specific heat of the rubber is 1650 J/(kg K), estimate the time required for the center of the rubber to reach 132°C.
GIVEN
• Tire suddenly exposed to steam on both sides
• Steam temperature (T?) = 149°C
• Tire thickness (2L) = 2.5 cm = 0.025 m
• Initial tire temperature (To) = 21°C
• The heat transfer coefficient (hc) = 150 W/(m2 K)
• The specific heat of the rubber (c) = 165 J/(kg K)
FIND
• The time required for the central layer to reach 132°C
ASSUMPTIONS
• Shape effects are negligible, tire can be treated as an infinite plate
SKETCH