Two crosslinkable polyesters are being considered for use as the resin in making a fiberglass-reinforced tank for processing various chemicals. One polyester is made from a glycol having four carbons between the alcohol groups and the other is made using the same acid but from a glycol having eight carbons between the alcohols. Compare the physical, chemical, and mechanical properties of the two
resins. If the chemicals being processed are water-based, which resin is the best candidate on the basis of chemical resistance?
What will be an ideal response?
The material containing the four carbons will have a higher crosslinked density and as such will have a higher shrinkage and stiffness and resistance to chemicals than the material containing the eight carbon bonds. For the same reasons as mentioned, the four carbon bonded polyester will have a better resistance to the water-based chemicals.
You might also like to view...
Why are there half as many teeth on the crankshaft drive as there are on the camshaft drive?
What will be an ideal response?
Coordinated resource management planning
What will be an ideal response?
Technician A says that with self-adjusting clutch systems, the release bearing constantly rotates. Technician B says the ball bearing portion of the release bearing should be lubricated with high temperature grease during routine maintenance. Which technician is correct?
A) Technician A only B) Technician B only C) Both technicians A and B D) Neither technician A nor B
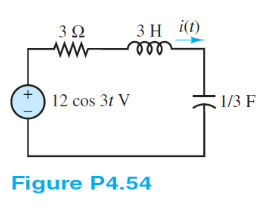
Use phasor techniques to solve for the current i(t) shown in Figure P4.54.