Use the pH scale to solve the problem. Negative ions, designated by the notation [OH-] are always present in any acid or base. The concentration, [OH-], of these ions is related to [H+] by the equation [OH-] ? [H+] = 10-14 moles per liter. Find the concentrations of [OH-] and [H+] in moles per liter for the substance with the following pH value. Round to two decimal places. pH = 12.3
A. [H+] = 2.00 × 10-13
[OH-] = 5.01 × 10-3
B. [H+] = 5.01 × 10-11
[OH-] = 2.00 × 10-3
C. [H+] = 5.01 × 10-13
[OH-] = 2.00 × 10-2
D. [H+] = 2.00 × 10-2
[OH-] = 5.01 × 10-13
Answer: C
You might also like to view...
Determine whether the following p-series is convergent or divergent.
?
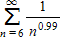
What will be an ideal response?
Solve the problem.A $154,000 trust is to be invested in bonds paying 7%, CDs paying 5%, and mortgages paying 10%. The bond and CD investment together must equal the mortgage investment. To earn a $12,470 annual income from the investments, how much should the bank invest in bonds?
A. $31,000 B. $77,000 C. $44,000 D. $46,000
Between each pair of numbers, insert the appropriate sign, <, =, or >, to make a true statement.1.3 1.45
A. 1.3 = 1.45 B. 1.3 > 1.45 C. 1.3 < 1.45
Multiply.
?

A. 2.03 B. 20.3 C. -20.3 D. -2.03 E. -5.73