Ideal Gas Law: On a cold day, you take in 4.2 L of air into your lungs at a temperature of 0°C. If you hold your breath until the temperature of the air in your lungs reaches 37°C, what is the volume of the air in your lungs at that point, assuming the pressure does not change?
A. 4.2 L
B. 4.4 L
C. 4.6 L
D. 4.8 L
E. 5.0 L
Answer: D
You might also like to view...
At what two celestial locations do the celestial equator and ecliptic coincide?
a. winter solstice and summer solstice b. vernal equinox and autumnal equinox c. they coincide at all points because they are the same. d. north celestial pole and south celestial pole e. zenith and east point
Some large clusters of galaxies do not appear to contain enough mass to hold themselves together
Indicate whether the statement is true or false
Molecular Rotation: In its lowest quantum state, a diatomic quantum mechanical rotator has a rotational energy of
A. zero.
B. 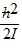 .
.
C. 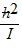 .
.
D. none of the given answers.
Where are open clusters found?
A.) No stars are forming in the galaxy today. B.) In the halo C.) In the disk. D.) Only around the Sun. E.) B and C.