A certain gas is described by the equation of state
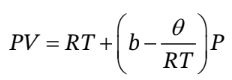
What will be an ideal response?
Here, b is a constant and ? is a function of T only. For this gas, determine expressions for the isothermal compressibility ? and the thermal pressure coefficient (?P /?T ) V . These expressions should contain only T, P, ?, d?/dT, and constants.
For this problem, we simply need to use the definitions of the isothermal compressibility and the thermal pressure coefficient, and take the specified partial derivatives. If the equation of state is:
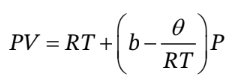
Then we can write V as a function of P and T as
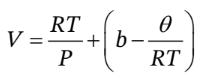
The definition of ? is
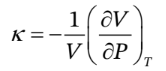
So, we just need to take the derivative of V with respect to P at constant T, which is
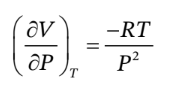
So,
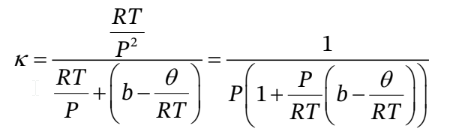
Similarly, to find the thermal pressure coefficient, we can solve the EOS for P in terms of T and V to get
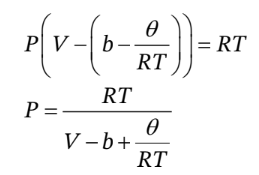
So, taking the derivative with respect to T at constant V, we have
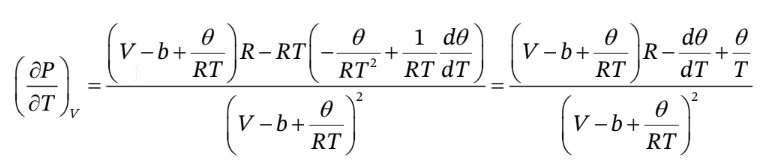
If we want this only in terms of T, P, ? and d?/dT, we should then substitute into it
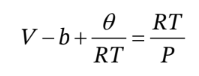
Note that we have to do this after taking the derivative, since we need to have V in the expression so that we can hold it constant while taking the derivative. Substituting this in gives
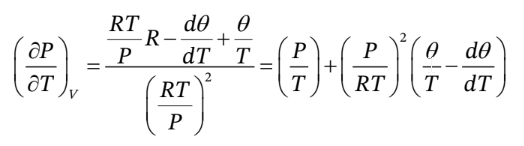
You might also like to view...
Which of the following describes MSCPs?
a. Insulating fibers mounted on a shaft b. Fuse-like devices designed for use only in a special type of fusible-switch combination motor starter c. Structures of copper or aluminum wire coils d. Circuit breakers suitable for use as a motor disconnecting means
A l-in.-diameter steel bar is subjected to a tensile force of 30 kips. Determine the stress in the bar.
What will be an ideal response?
Determine the number of times interest is compounded in a year for the following interest statements: (a) 1% per quarter; (b) 2% per month; and (c) 8% per year, compounded every 2 months.
What will be an ideal response?
Why is the control of obsolescence and knowledge of what parts are selling or not in the parts department so important?
What will be an ideal response?