Ideal Gas Law: Consider two equal-volume flasks of gas at the same temperature and pressure. One gas, oxygen, has a molecular mass of 32. The other gas, nitrogen, has a molecular mass of 28. What is the ratio of the number of oxygen molecules to the number of nitrogen molecules in these flasks?
A. 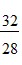
B. 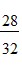
C. 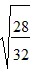
D. 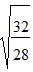
E. 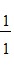
Answer: E
You might also like to view...
In a binary system containing two neutron stars, the objects will ____
a. radiate orbital energy away as gravitational radiation and move farther apart b. radiate orbital energy away as gravitational radiation and move closer together c. exchange mass until their orbital periods become equal d. exchange mass until they are in synchronous rotation e. exchange angular momentum, lengthening the period of the pulsar
Exclusion Principle: If ? = 4, which one of the following is a possible quantum number for n?
A. 0 B. 2 C. 3 D. 4 E. 8
Escape Speed: Planet A has twice the mass of Planet B. From this information, what can we conclude about the escape speed for Planet A compared to that of Planet B?
A. The escape speed for Planet A must be twice as great as the escape speed from Planet B. B. The escape speed for Planet A must be four times as great as the escape speed from Planet B. C. The escape speed for Planet A is the same as the escape speed from Planet B. D. The escape speed for Planet A is greater than the escape speed from Planet B, but we cannot say how much greater. E. We cannot conclude anything about the escape speed for Planet A without knowing the radii of the two planets.
Emission lines from different ionization states of the same element appear in the same place in the spectrum
Indicate whether the statement is true or false