Oxygen gas expands in a flexible container following a relationship of PV1.15 = constant. The mass of the oxygen is 1.75 lbm, and initially the pressure and temperature of the oxygen is 150 psi and 150oF, respectively. The expansion continues until the volume is double the original volume. Determine the final pressure and temperature of the oxygen, and the work done by the oxygen during the expansion.
Given: m = 1.75 lbm; T1 = 150oF = 610 R; P1 = 150 psi = 21,600 lbf/ft2; V2 = 2V1; PV1.15 = constant.
What will be an ideal response?
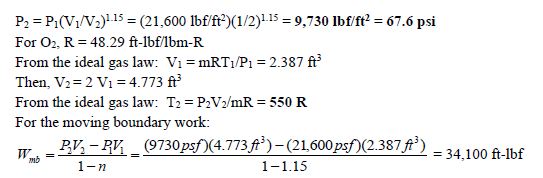
You might also like to view...
Discuss five skills that are important to a leader during crisis management.
What will be an ideal response?
Consider two atoms: one red and the other blue. There are six locations to place those two atoms. You can only have one atom in each location. How many microstates exist for this system?
A. 15 B. 30 C. 36 D. 12 E. None of the above
Which of the following is administered as an agency of the U.S. Department of Agriculture?
a. Federal land banks c. Small Business Administration b. Commodity Credit Corporation d. Production Credit Associations
Bioremediation is an example of a service that an ecosystem can provide
Indicate whether the statement is true or false