An aqueous solution has a hydrogen concentration [H+] of 0.3 mol/L. Find the concentration of hydroxl ions [OH-] in the solution.
A. 3.33 × 1014 mol/L [H+]
B. 3.33 × 10-14 mol/L [H+]
C. 3.33 mol/L [H+]
D. 3.33 × 10-13 mol/L [H+]
Answer: B
Mathematics
You might also like to view...
Subtract. Combine like terms, and write the answer in descending order.(7z + 5z2 + 6) - (10z2 - 1)
A. -5z2 + 8z + 6 B. 15z2 + 7z + 5 C. -5z2 + 7z + 7 D. 15z2 + 8z + 6
Mathematics
Solve the equation.10n = 4n + 6 + 5n
A. -6 B. -60 C. 6 D. 60
Mathematics
Add.(-2x2 + 8x) + (10x2 - 6x - 5)
A. 8x4 + 2x2 - 5 B. 10x6 - 5 C. 8x2 + 2x + 5 D. 8x2 + 2x - 5
Mathematics
Calculate.-  2
2
A. -100
B. - 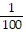
C. 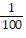
D. 100
Mathematics