How much heat is required to change 456 g of ice at -25.0°C into water at 25.0°C? The specific heat of ice is 2090 J/(kg K) and the latent heat of fusion of water is 33.5 × 104 J/kg
A) 224 kJ
B) 153 kJ
C) 112 kJ
D) 71.5 kJ
E) 72.5 kJ
A
You might also like to view...
While observing the Sun, you note a large number of sunspots. What can you conclude?
A) The Sun is less luminous than usual. B) This is a period of low solar activity. C) Earth's climate will be unusually cold. D) The Sun's rotation is slower than average. E) There are likely to be an above average number of flares and prominences.
A planar cross section through two spherical waves emanating from the sources S1 and S2 in the plane is shown in the figure. The black circles are one and two wavelengths from their respective sources. The lighter circles are one half and one and a half wavelengths distant from their respective sources. If the phase at S1 and S2 is zero at this instant, and the waves shown arriving at P1 both arrive with amplitude A, the magnitude of the phase angle of each wave at point P1 (in radians) is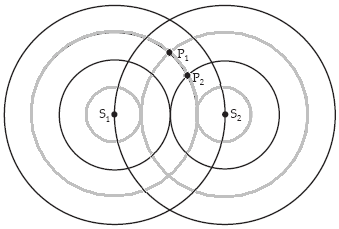
A. 0. B. ?. C. 2?. D. 3?. E. ?/2.
How much kinetic energy must a deuterium ion (charge 1.6 × 10^?19 C) have to approach to within 10?14 m of another deuterium ion? (1 MeV = 1.6 × 10^?13 J)
A heat engine having the maximum possible efficiency has an efficiency of 25% when operating between two heat reservoirs. If the temperature of the cold reservoir is 300 K, what is the temperature of the hot reservoir?
A) 375 K B) 450 K C) 400 K D) 350 K E) 500 K