Carbon dioxide has a pressure of 1500 kPa. Using both the ideal gas law and van der Waals equation, determine the specific volume of the CO2 for temperatures of (a) 250 K, (b) 500 K, and (c) 2000 K.
Given: CO2, P = 1500 kPa
What will be an ideal response?
For CO2, R = 0.1889 kJ/kg-K; Tc = 304.2; Pc = 7,390 kPa
From the ideal gas law: v = RT/P
For van der Waals Equation, we need the constants a and b:
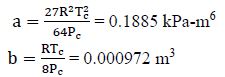
van der Waals equation: 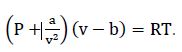
(a) T = 250 K
Ideal gas law: v = 0.03148 m3/kg
van der Waals equation: v = 0.02815 m3/kg
(b) T = 500 K
Ideal gas law: v = 0.0630 m3/kg
van der Waals equation: v = 0.0619 m3/kg
(c) T = 2000 K
Ideal gas law: v = 0.2519 m3/kg
van der Waals equation: v = 0.2523 m3/kg
You might also like to view...
When machine grinding valves, which of the following specifications requires close observation?
A. fillet B. valve protrusion C. valve recession D. margin
________ are plant hormones derived from amino acids (ornithine) that are important in plant developmental processes that involve cell division.
Fill in the blank(s) with the appropriate word(s).
Adventitious and seminal roots make up what part of the grass plant?
a. head c. root b. bottom d. shoot
How has international trade improved the availability of food in the United States and around the world?
What will be an ideal response?