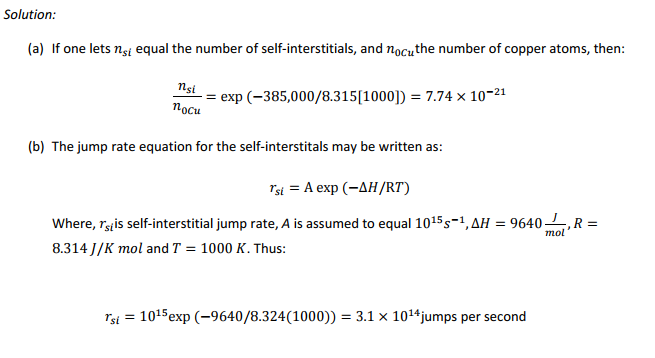
(a) It has been estimated that the enthalpy for the formation of self?interstitial atoms in copper is about 385,000 J/mol. Compute the equilibrium concentration of these interstitial atoms in copper at 1000K.
(b) The activation energy for the movement of the self?interstitials atoms in copper is believedto be about 9,610 J/mol. Estimate the jump frequency of the interstitials at 1000K.
You might also like to view...
Efficiency is the ratio of the unused power output to the total power input.
Answer the following statement true (T) or false (F)
A ring of mulch around the base of a tree can protect it from damage during mowing
Indicate whether the statement is true or false
We know about the Greek rulers of Bactria and India primarily from
a. the writings of Greek philosophers these rulers patronized. b. documents in Sanskrit describing the conversion of these rulers and their nobles to Hinduism. c. coins and popular tales. d. monumental architecture, especially their palaces and temples to Greek gods.
The kick block, or kicker, is placed on the lower floor supporting the stair.
Answer the following statement true (T) or false (F)