Air enters a diffuser with a pressure of 200 kPa, a temperature of 50oC, and a velocity of 250 m/s. The inlet of the diffuser is circular, with a diameter of 2.5 cm. The exit velocity is 20 m/s. Plot the exit temperature for heat transfer rates ranging from -20.0 kW to 20.0 kW. Repeat the calculations for an outlet velocity of 100 m/s.
Given: Air, P1 = 200 kPa; T1 = 50oC = 323 K; V1 = 250 m/s; D1 = 2.5 cm = 0.025 m; V2 = 20 m/s; and V2 = 100 m/s
What will be an ideal response?
For a diffuser, assume steady –state, steady-flow single-inlet, single-outlet flow, and assume that W?=?pe=0
These assumptions result in the first law being (State 1 is inlet, State 2 is outlet)
As the temperature change is likely small, we will consider air to be an ideal gas with constant specific heats: h2 – h1 = cp (T2 – T1)
A1 = ?D12/4 = 0.0004908 m2
m?=?1V1A1=P1RT1V1A1 = 0.2648 kg/s
where R = 0.287 kJ/kg-K. For air, take cp = 1.005 kJ/kg-K
Substituting into the First Law:
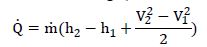
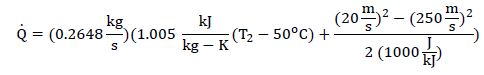
Notice, with the specified conditions, there is no outlet pressure dependency
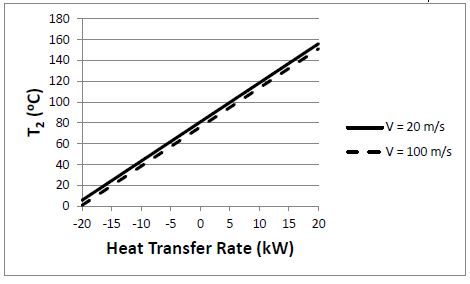
The plot below compares the results for the two outlet velocities.
You might also like to view...
A colorless, odorless, tasteless gas that supports combustion is _____.
a. helium b. oxygen c. nitrogen d. neon
What is sprinkler irrigation often used for?
What will be an ideal response?
What is much of the treatment of the separately derived system is concentrated in?
What will be an ideal response?
Coprophagy
What will be an ideal response?