A large calorie (Cal) is the amount of heat needed to change the temperature of one ______________ of water by one Celsius degree
a. dekagram
b. centigram
c. gram
d. kilogram
d
You might also like to view...
Thermodynamic Devices: During each cycle, a refrigerator removes 20.0 kJ of heat from the freezing compartment and ejects 24.0 kJ into a room.(a) How much work per cycle is required each cycle to run this refrigerator?(b) What is the coefficient of performance of this refrigerator?
Fill in the blank(s) with the appropriate word(s).
A runner runs on a circular path of radius 10 m. What is the magnitude of the displacement of the jogger if he runs (a) half-way around the track? (b) all the way around the track?
What will be an ideal response?
Schroedinger discovered
A) a way to predict the wave patterns seen in experiments involving matter waves. B) the particle theory of radiation. C) the probabilistic interpretation of matter waves. D) the proper way to precisely predict the behavior of individual sub-atomic particles. E) the fact that electrons sometimes behave like waves.
The figure shows a ‘snapshot’ of four different waves in identical ropes stretched with the same force
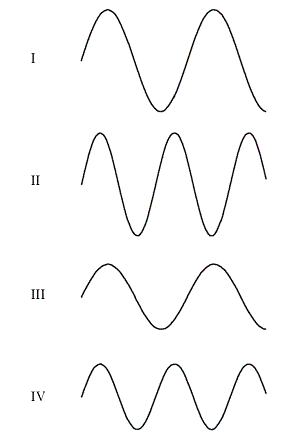
The wave with the largest wavelength is wave
a.
I.
b.
II.
c.
III.
d.
IV.
e.
all of the above