Silicon doped with arsenic produces an n-type semiconductor with a donor level at 0.049 eV below the conduction band in an energy gap of 1.1 eV.
(a) What is the wavelength of the lowest energy absorption edge for this semiconductor?
(b) To what region of the electromagnetic spectrum does this wavelength correspond?
(a) The lowest energy absorption edge corresponds to excitation of donor electrons into the conduction band requiring an energy of 0.049 eV. The excitation from the valence band to the conduction band requires photons equal to the energy gap of 1.1 eV, and this is a much higher energy. Use Equation 18.2 to solve for the wavelength.
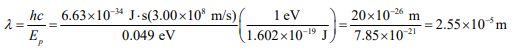
(b) this wavelength is in the infrared portion of the spectrum.
You might also like to view...
Neglecting air resistance, a ball projected straight upward so it remains in the air for 10 seconds needs an initial speed of
A) 50 m/s. B) 60 m/s. C) 80 m/s. D) 100 m/s. E) 110 m/s.
How far above or below the ecliptic can the Sun move?
What will be an ideal response?
How can close analysis of the spectra of quasars let us map the whole universe?
What will be an ideal response?
The only force acting on a 1.8-kg body as it moves along the x axis is given by Fx = -(3.0x) N, where x is in m. If the velocity of the body at x = 0 is vx = +8.0 m/s, at what value of x will the body have a velocity of +4.0 m/s?
a. 5.7 m b. 5.4 m c. 4.8 m d. 4.1 m e. 6.6 m