What does the phenomenon of diffraction demonstrate?
A) the wave nature of light
B) the polarization of light waves
C) the quantization of atomic orbitals
D) the particle nature of the photon
E) the process of ionization
A
You might also like to view...
Which of the partition functions below yields an average energy Eavg that is NOT an integer multiple of 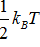 in the high-temperature (small-?) limit? Assume that ? is a constant with units of energy, and ? = 1/kBT.
in the high-temperature (small-?) limit? Assume that ? is a constant with units of energy, and ? = 1/kBT.
A. Z = (2?/??)2
B. Z = (??)?1/2
C. Z = (1 ? e??? )?1 (note that ex ? 1 + x when 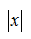 << 1)
<< 1)
D. None of the above.
E. All of the above.
In analyzing electric circuits containing a battery and at least one resistor, what is the change in potential across a resistor as one moves through it in the direction of the current?
a) +i²R b) -i²R c) +iR d) -iR e) zero
Water surrounds hot horizontal tubes 3 cm in diameter in a nuclear steam generator having surface temperature of 800ºC. The water is at a pressure of 800 kPa. If film boiling is assumed, estimate the total convection heat transfer coefficient between the water and the tube surfaces.
What will be an ideal response?
Observations show that elements with atomic mass numbers divisible by 4 (such as oxygen-16, neon-20, and magnesium-24) tend to be more abundant in the universe than elements with atomic mass numbers in between. Why do we think this is the case?
A) At the end of a high-mass star's life, it produces new elements through a series of helium capture reactions. B) The apparent pattern is thought to be a random coincidence. C) Elements with atomic mass numbers divisible by 4 tend to be more stable than elements in between. D) This pattern in elemental abundances was apparently determined during the first few minutes after the Big Bang.