The top of a 30 cm I-beam is maintained at a temperature of 260°C, while the bottom is at 93°C. The thickness of the web is 1.25 cm. Air at 260°C is blowing along the side of the beam so that h= 40 W/(m2 K). The thermal conductivity of the steel may be assumed constant and equal to 43 W/(m K). Find the temperature distribution along the web from top to bottom and plot the results.
GIVEN
FIND
The temperature distribution along the web and the plot the results
ASSUMPTIONS
The thermal conductivity of the steel is uniform
The beam has reached steady state conditions
One dimensional through the web
The beam is very long compared to the web thickness
SKETCH
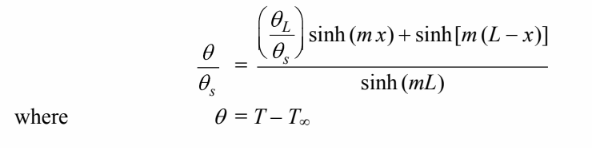
The web of the I beam can be thought of as a fin with a uniform rectangular cross section and a fixed tip
temperature the temperature distribution along the web is
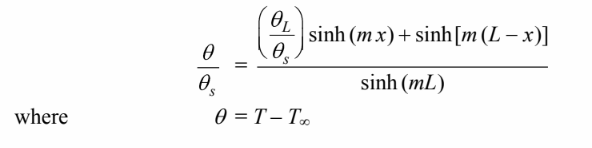
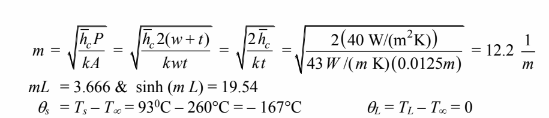
Substitute these into the temperature distribution

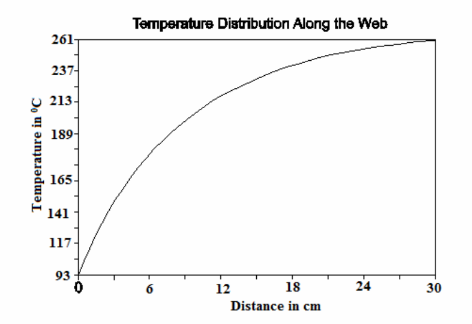
This temperature distribution is plotted below
You might also like to view...
Springs: An object attached to a spring is pulled across a horizontal frictionless surface. If the force constant (spring constant) of the spring is 45 N/m and the spring is stretched by 0.88 m when the object is accelerating at 1.4 m/s2, what is the mass of the object?
A. 28 kg B. 24 kg C. 31 kg D. 36 kg
Two glass plates, each with an index of refraction of 1.55, are separated by a small distance D. The space between the plates is filled with water (n = 1.33) as shown. What is the minimum thickness of D that will cause the reflected light appear green? Note: The wavelength of green light is 460 nm in vacuum.
A particular object appears to be red. The reason it appears red is:
1.It absorbs only red frequencies as they hit. 2.It reflects only red frequencies as they hit. 3.It absorbs red frequencies more than other frequencies. 4.It reflects red frequencies more than other frequencies. 5.It emits red frequencies more than other frequencies. 6.Not enough information.
The percentage by mass of chlorine
a. would be the same in FeCl3 as in FeCl2. b. would be greater in FeCl3 than in FeCl2. c. would be less in FeCl3 than in FeCl2. d. cannot be determined without additional information.