A hydrogen atom is excited to the n = 9 level. Its decay to the n = 6 level detected in a photographic plate. What is the frequency of the light photographed? (1 eV = 1.60 × 10-19 J, h = 6.626 × 10-34 J ? s)
A) 5910 nm B) 5910 Hz C) 5.08 × 1013 Hz D) 3.28 × 10-9 km
C
You might also like to view...
During a total lunar eclipse,
a. the Moon must be new. b. the observer must be in the path of totality. c. the Moon's color will be affected by Earth's atmosphere. d. the Moon must be at about its greatest distance from Earth. e. it must be near the time of one of the equinoxes.
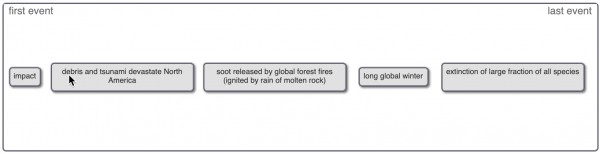
Put the following events into the correct order in which they are thought to have occurred according to the impact hypotethesis.
Why do you think you were asked to complete more cycles in Procedure 1, Step 3, if the total time was less than 10 seconds for 10 cycles?
For experiments involving the setup shown in figure Q4.1b, which of the following possible results (if seen) regarding the final value displayed by the voltmeter would probably not be consistent with the wave model of light? 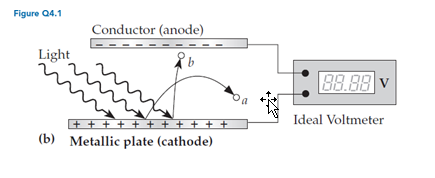
A. It increases as the light’s intensity increases. B. It is independent of the light’s intensity. C. It varies as the light’s wavelength changes. D. It increases as the rate of electron ejection increases.