Generally, along the polar front one would not expect to observe:a
temperatures on one side lower than on the other side.
b. an elongated region of lower pressure.
c. clouds and precipitation.
d. converging surface air.
e. sinking air aloft.
e
You might also like to view...
Why is calcium fluoride, CaF2, a high melting point crystalline solid while stannic chloride, SnCl4, is a volatile liquid?
A) There is no theory to predict the physical property of melting point. Melting point temperatures are empirically determined. B) Actually, we would predict these results to be the opposite. Since each metal is combined with a group 17 halogen, the heavier metal (tin) combination should have the higher melting point. C) CaF2 is a small, linear, non-polar molecule, while SnCl4 is a huge tetrahedral structure. Therefore the bonds in calcium fluoride tend to give it a higher melting point temperature. D) Ionic compounds formed by elements on opposite sides of the periodic table, like CaF2, tend to have higher melting points than more covalently bonded structures, like SnCl4.
Which of the following is shown as a heavy blue line with triangles?
A) dry line B) warm front C) cold front D) occluded front
What is the difference between a flowing artesian well and a nonflowing artesian well?
What will be an ideal response?
This cross section shows the location of a septic tank with harmful bacteria and 5 wells. Which well is least likely to become contaminated?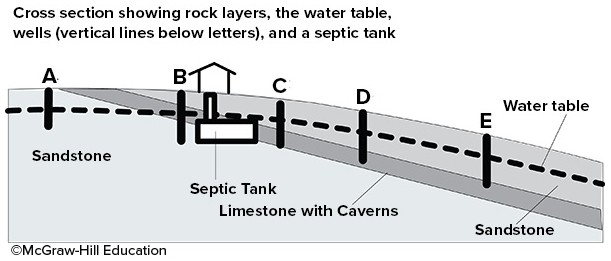
A. well A B. well B C. well C D. well D E. well E