The spectral lines of each element are distinctive to that element, whether we are looking at emission or absorption lines
Indicate whether the statement is true or false
TRUE
You might also like to view...
In order of visual luminosity at the start, which is most luminous?
A) a red supergiant B) a planetary nebula C) a nova D) a type I supernova E) a type II supernova
Consider the planet Mars: [Earth mass = 6.0 × 1024 kg = 9.3 × Mars mass; Earth radius = 6380.km = 1.89 × Mars radius] What is the acceleration of gravity on Mars?
What will be an ideal response?
Two electrons are placed into separate (very tiny) one-dimensional boxes A and B. When the electrons are excited to higher energy levels and then decay to lower levels, they emit photons. The longest-wavelength spectral line from the electron in box A is observed to have the same wavelength as the third-longest spectral line from the electron in box B. The ratio of the box lengths LA/LB is equal to
A. 3/7
B. 7/3
C. 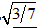
D. 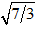
E. 9/49
State the principle of superposition
What will be an ideal response?