How much heat must be removed from 456 g of water at 25.0°C to change it into ice at -10.0°C? The specific heat of ice is 2090 J/(kg K) and the latent heat of fusion of water is 33.5 × 104 J/kg
A) 105 kJ
B) 153 kJ
C) 57.3 kJ
D) 47.7 kJ
E) 210 kJ
E
You might also like to view...
What is the density of a mixture of 85% air 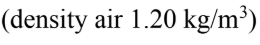 and 15% water vapor (density of water vapor
and 15% water vapor (density of water vapor 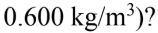
A. 1.70 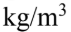
B. 1.87 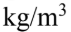
C. 1.05 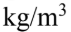
D. 1.11 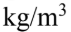
E. 1.19 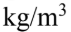
The process where surface waters are pushed away from the land and replaced by nutrient-rich bottom waters is called
A) downwelling. B) upwelling. C) land breeze. D) sea breeze.
What did Galileo discover through his telescope when he looked at Jupiter, and how did it refute the Ptolemaic model?
What will be an ideal response?
A tennis ball is held above and in contact with a basketball, and then both are simultaneously dropped. The tennis ball bounces off the basketball at a fairly high speed. This is because:
a. the basketball falls farther than the tennis ball. b. the tennis ball is slightly shielded from the Earth's gravitational pull. c. the massive basketball transfers momentum to the lighter tennis ball. d. the tennis ball has a smaller radius.