Which of the following is a consequence of the laws of thermodynamics?
A.Heat can be completely changed into work
B.Heat transfers from a cold object to a hot object until equilibrium is reached
C.Atoms can be at any temperature, even absolute zero
D.Work can be completely changed into heat
Work can be completely changed into heat by the second law of thermodynamics. Heat engines with 100% efficiency are prohibited, but the prohibited part is changing heat into work. To understand this example, imagine dragging a box along the ground with a constant force. The work put into the system accelerates the box against the force of friction, but eventually friction slows the box to a complete stop. The friction basically turns the energy of the box into heat.
You might also like to view...
The wave functions of some molecules are a combination of wave functions with different values of the orbital quantum number . The wave function of PF5 combines s, p and d states in an sp3d hybrid orbital. We would expect such an overlap of wave functions in individual molecules to represent
a. ionic bonding b. metallic bonding. c. covalent bonding. d. Van der Waal's bonding. e. hydrogen bonding.
The__________is the spiral galaxy that contains the Sun
A) Andromeda Galaxy B) Cartwheel Galaxy C) Milky Way Galaxy D) Pinwheel Galaxy
What is the total energy stored in the group of capacitors shown if the charge on the 30-?F capacitor is 0.90 mC?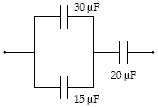
A. 29 mJ B. 61 mJ C. 21 mJ D. 66 mJ E. 32 mJ
Equation of Traveling Waves: The vertical displacement y(x,t) of a horizontal string aligned along the x-axis is given by the equation y(x,t) = (6.00 mm) cos[(3.25 m-1)x - (7.22 s-1)t]. What are the (a) wavelength and (b) period of this wave?
What will be an ideal response?