The figure below provides a Pxy diagram for the R-134a (1) + R-245fa (2) system at 293.15 K. Which of the following is true?
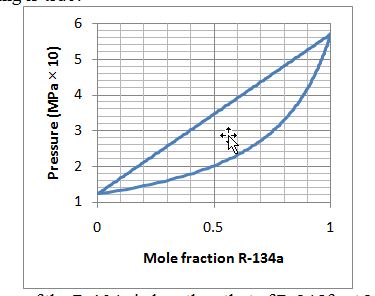
A. The vapor pressure of the R-134a is less than that of R-245fa at 293.15 K.
B. At 293.15 K and 0.4 MPa, an equimolar mixture of this system will exist in two phases (liquid and vapor).
C. At 293.15 K and 0.3 MPa, a mixture that contains 20 moles of R-134a and 80 moles of R-245fa will exist in a single liquid phase.
D. At 293.15 K and 0.2 MPa, a mixture that contains 80 moles of R-134a and 20 moles of R-245fa will exist in a single liquid phase.
E. None of the above are true.
A. Incorrect. This is backwards. Remember that, although the abscissa is labeled in R-134a, at x = 0, none is present and we read the vapor pressure of the other component (R-245fa).
B. Incorrect. Find the point on the graph indicated; it is above the bubble-point curve and, thus, will exist as one liquid phase.
C. Correct. This point is above the bubble-point curve on this Pxy plot, so we know it will exist as one liquid phase.
D. Incorrect. This point is under the dew-point curve, so we will have one vapor phase.
E. Incorrect. One of the statements is true.
You might also like to view...
What is the difference between shallow foundations and deep foundations? Where would you recommend the use of deep foundations? Explain.
What will be an ideal response?
The organization(s) that test and list circuit breakers is/are _____.
a. IEEE b. OSHA and NEMA c. CSA and UL d. UL and NEMA
The Miniature Schnauzer is of ____________________ origin and dates back to the fifteenth century
Fill in the blank(s) with correct word
Because CFL bulbs contain mercury that is harmful to humans and the environment, they should not be used to replace incandescent light bulbs.
Answer the following statement true (T) or false (F)