Suppose that the liquid molar enthalpy (H) for a binary mixture of benzene (1) and cyclohexane (2) has been fitted to the following functional form (at 50 °C and 9.4 bar)
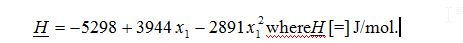
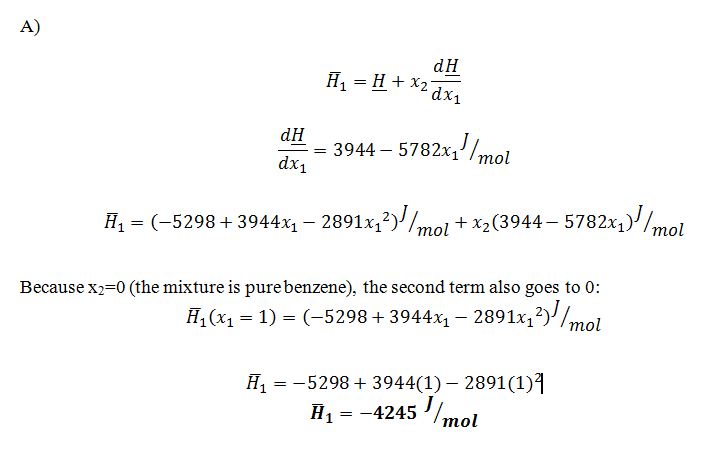
A) What is the partial molar enthalpy, ¯H_1, of benzene when x1 = 1 at 50°C and 9.4 bar?
B) What is the partial molar enthalpy of benzene in this mixture (at this T and P) when it is equimolar?
C) What is the partial molar enthalpy of cyclohexane in this mixture (at this T and P) when it is equimolar?
D) What is the pure component enthalpy of cyclohexane at this temperature and pressure?

You might also like to view...
A greenhouse or forestry plot may serve as an agricultural lab
Indicate whether the statement is true or false
What are some advantages to raising large game animals?
What will be an ideal response?
Compared to gypsum, elemental sulfur is sometimes considered a more desirable fertilizer material because:
A) it causes less soil acidity than gypsum. B) plants respond to sulfur more rapidly. C) it is less dependent on warm, moist conditions for availability. D) it contains more nutrient sulfur, kilogram for kilogram, than gypsum. E) all of the above
Of the various types of tungsten electrodes available, the tungsten electrodes that are used for AC welding are
_____. a. lanthanated b. pure c. irradiated d. thoriated