Neon has constant specific heats whose ratio is 1.667. The molecular mass of neon is 20.183 kg/kmole. Determine the values of cp and cv for neon.
Given: k = cp/cv = 1.667; M = 20.183kg/kmole
What will be an ideal response?
First, find R for Neon: 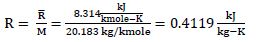
For an ideal gas, cp – cv = R, so cp = R + cv
Substituting: k = (R+cv) / cv = 1.667 = (0.4119 kJ/kg-K + cv)/cv
Solving: cv = 0.618 kJ/kg-K
Then, cp = R + cv = 1.029 kJ/kg-K
You might also like to view...
Which is an oversell? The service consultant ____________
A. Sells an oil change after the internal engine repairs are completed B. Sells snow tires in winter to replace regular tires that are good C. Sells an oil change at 1000 miles when the service requirement is 5000 miles D. Sell a new designed wipe blade that works better than standard wiper blade
What flows in a conductor to produce a magnetic field proportional to the current is produced?
A. Amps B. Resistance C. Volts D. Current
Coolant pump leaks are often caused by worn bearings.
Answer the following statement true (T) or false (F)
ASE-Style Multiple Choice While having a discussion, Technician A says that computer memory is the portion of the computer that directs the sequence of operations by electrical signals and governs the actions of the units that make up the computer. Technician B says that the system software is responsible for these activities. Who is correct?
A. A only B. B only C. Both A and B D. Neither A nor B