What is the value of P that makes the work of the two processes the same?
A process consists of two steps: (1) One mole of air at T = 800 K and P = 4 bar is cooled at constant volume to T = 350 K. (2) The air is then heated at constant pressure until its temperature reaches 800 K. If this two-step process is replaced by a single isothermal expansion of the air from 800 K and 4 bar to some final pressure P.
Assume mechanical reversibility and treat air as an ideal gas with 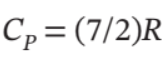 and
and 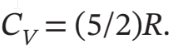
The initial volume of 1 mol of air at 800 K and 4 bar (400000 Pa) is V = RT/P = 8.3145 * 800 / 400000 = 0.01663 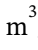 . When it is cooled at constant volume to 350 K, the pressure drops to 4 bar * 350 K/800 K = 1.75 bar = 175000 Pa. No work is done during this constant volume cooling step. When it is heated back to 800 K at constant pressure, the volume increases to is V = RT/P = 8.3145 * 800 / 175000 = 0.03801
. When it is cooled at constant volume to 350 K, the pressure drops to 4 bar * 350 K/800 K = 1.75 bar = 175000 Pa. No work is done during this constant volume cooling step. When it is heated back to 800 K at constant pressure, the volume increases to is V = RT/P = 8.3145 * 800 / 175000 = 0.03801 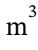 The work done on the gas is -P?T = -175000 Pa *
The work done on the gas is -P?T = -175000 Pa * 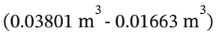 = -3742 J. So, the work done by the gas is 3742 J.
= -3742 J. So, the work done by the gas is 3742 J.
For an isothermal expansion, the work will be 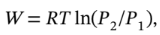 or
or 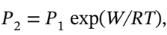 or
or
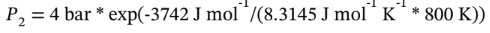 = 2.27 bar.
= 2.27 bar.
So, the isothermal expansion does the same amount of work with less expansion of the gas.
You might also like to view...
Although the starting points for the three lines will be different, the _____ for all three lines in an equal-spread offset is always the same.
a. set and travel b. diameters c. ending points d. branches
Forestry is considered a(n) ____________________ industry
Fill in the blank(s) with correct word
Which is NOT a function of an ultracapacitor?
A) Can pass AC current B) Can pass DC current C) Can be charged with DC current D) Discharges DC current
What is the cofunction of the tangent of 34° 9'?
What will be an ideal response?