A substance consisting of which molecule shown below should have a higher boiling point?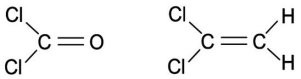
A. The molecule on the right, C2H2Cl, because it has a greater number of atoms.
B. The molecule on the left, COCl2, because it has a fewer number of atoms.
C. The molecule on the right, C2H2Cl, because of electronegativity considerations.
D. The molecule on the left, COCl2, because it is less symmetrical.
Answer: C
You might also like to view...
Conditions in oceanic trenches that are inhospitable to life include
A. lack of light, extreme cold, and toxic chemicals. B. lack of light, extreme cold, high pressure, and scarce food. C. lava, high pressure, and scarce food. D. lack of light, extreme heat, and high pressure.
Which of the following events is not associated with climate change?
A. a volcanic eruption B. an earthquake over magnitude 8.0 C. an increase in the level of water vapor in the atmosphere D. deforestation
The temperature on a cloudy night is likely to be ________ the temperature on a clear night—all other factors being equal
A) warmer than B) colder than C) the same as
What is a common, average value for the angle of repose?
a. 3º b. 13º c. 33º d. 63º