What is the relationship between Bohr's model and the characteristic spectral lines of gases?
a. None. Bohr's model does not explain spectral lines.
b. The spectral lines are determined by the size of the initial orbit.
c. The spectral lines are determined by the various orbital jumps electrons can make.
d. The spectral lines are determined by the absorption of photons by the atom.
e. The spectral lines are determined by the angles at which electrons are emitted when light hits them.
c
You might also like to view...
Why is iron significant to understanding how a supernova occurs?
A) Iron is the heaviest of all atomic nuclei, and thus no heavier elements can be made. B) Supernovae often leave behind neutron stars, which are made mostly of iron. C) The fusion of iron into uranium is the reaction that drives a supernova explosion. D) Iron cannot release energy either by fission or fusion.
Life emerged on Earth within about a billion years after the solar system formed
Indicate whether the statement is true or false
The total linear momentum of an isolated system of masses remains the same if there are no external ______________ acting on the system
a. forces b. torques c. unbalanced forces d. unbalanced torques
Which of the six objects shown below has the largest rotational energy? (All have the same mass.)
A. 
B. 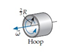
C. 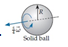
D. 
E. 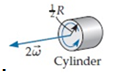
F.