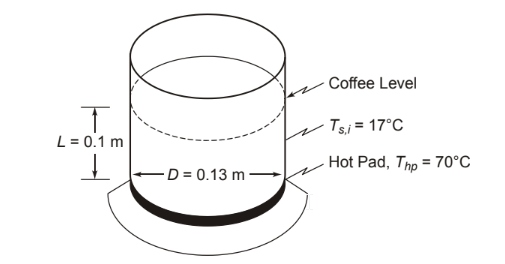
A plot of coffee has been allowed to cool to 17°C. If the electrical coffee maker is turned back on, the hot plate on which the pot rests is brought up to 70°C immediately and held at that temperature by a thermostat. Consider the pot to be a vertical cylinder 130 mm in diameter and the depth of coffee in the pot to be 100 mm. Neglect heat losses from the sides and top of the pot. How long will it take before the coffee is drinkable (50°C)? How much did it cost to heat the coffee if electricity costs $0.05 per kilowatt-hour?
GIVEN
• Coffee pot, idealized as a vertical cylinder, on a hot plate
• Initial temperature of the pot and coffee (Ts,i) = 17°C
• Hot plate temperature (Thp) = 70°C (constant)
• Pot diameter (D) = 130 mm = 0.13 m
• Depth of coffee (?) = 100 mm = 0.1 m
FIND
(a) Time for the coffee to reach 50°C (b) Cost to heat the coffee if electricity costs $0.05/kWh
ASSUMPTIONS
• Heat losses from the sides and the top are negligible
• All energy from the hot plate goes into the coffee
• Internal resistance of the coffee is negligible
• Thermal resistance of the bottom of the pot is negligible
• Coffee has the thermal properties of water
• Variation of the thermal properties of the coffee with temperature can be neglected
SKETCH
PROPERTIES AND CONSTANTS
The relevant thermal properties will be evaluated using the average coffee temperature of (17°C + 50°C)/2 = 33.5°C.
for water At 33.5°C
Density (?) = 994.6 kg/m3
Specific Heat (c) = 4175 J/(kg K) At the mean temperature of (33.5°C + 70°C)/2 = 51.8°C
Thermal expansion coefficient (?c) = 0.00047 1/K Thermal conductivity (kc) = 0.648 W/(m K) Kinematic viscosity (?c) = 0.549 × 10–6 m2/s Prandtl number (Prc) = 3.5
(a) The heat transfer coefficient from between the hot plate and the coffee can be evaluated by treating the coffee volume as a horizontal water layer heated from below. The Rayleigh number is

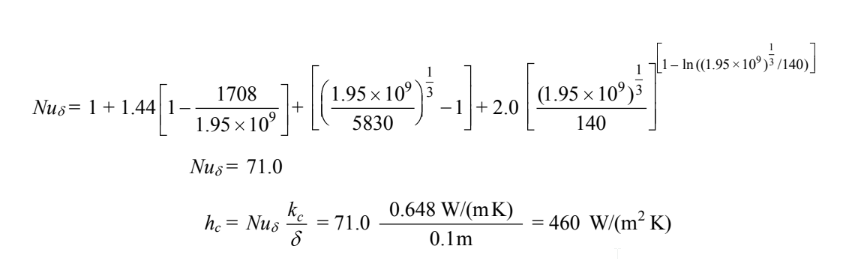
The Nusselt number for this geometry is
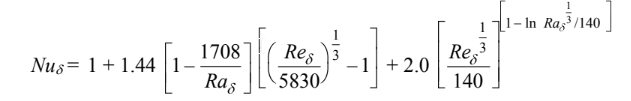
where the notation [ ] indicates that if the quantity inside the bracket is negative, the quantity is to be taken as zero.
The time required for heating can be calculated solving for the time
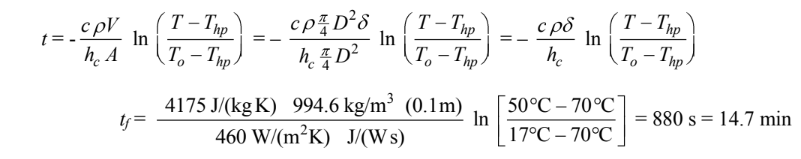
(b) The total heat transfer from the hot plate during this time is
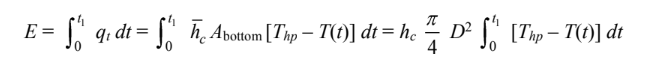
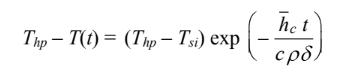
Therefore
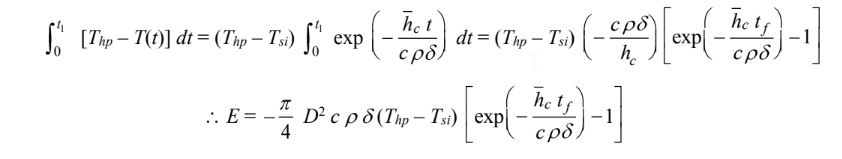
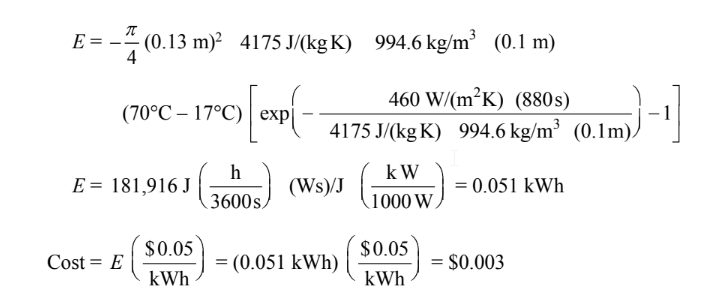
COMMENTS
The power consumption of the hot plat is about 12.5 watts.
The cost estimate neglects all losses from the hot plate to the ambient air.
You might also like to view...
What phase of the Moon must it be to have a lunar eclipse?
A. full moon B. first quarter C. new moon D. third quarter
The principle of equipartition of energy states that the internal energy of a gas is shared equally:
A. among the molecules B. between kinetic and potential energy C. among the relevant degrees of freedom D. between translational and vibrational kinetic energy E. between temperature and pressure
Two simple pendulums of equal length l = 0.45 m are suspended from the same point. The pendulum bobs are small solid steel spheres. The first bob is drawn back to make a 35° angle with the vertical, while the other one is left hanging at rest
If the first bob has a mass of 0.25 kg and the second has a mass of how high will the second bob rise above its initial position when struck elastically by the first bob after it is released? A) 2.7 cm B) 3.3 cm C) 4.4 cm D) 3.9 cm
Energy Levels: What is the energy of the photon emitted when an electron drops from the n = 20 state to the n = 7 state in a hydrogen atom?
A. 0.244 eV B. 0.263 eV C. 0.283 eV D. 0.302 eV