In practice, which of the following systems is the most likely to be modeled with the virial equation of state?
A. Benzene at STP
B. Ethanol at 150 K and 101 325 Pa
C. Propane at 100 Pa and 37 °C
D. Carbon dioxide at 500 kPa and 50 °C
E. Freon-22 at 400 K and 4 MPa
A. Incorrect. Benzene is a liquid at STP (273.15 K and 0.1 MPa), the virial EOS should only be used for vapors far from the critical point.
B. Incorrect. At these conditions ethanol is a SOLID; the virial EOS should only be used for vapors far from the critical point and few EOSs are designed to model the solid phase.
C. Incorrect. At this pressure the ideal gas law should be very accurate, and there would be no need to use a more complex equation.
D. Correct. Carbon dioxide is a gas at these conditions and far from the critical point.
E. Incorrect. Freon-22’s critical pressure is 4.97 MPa, to which this is very close. The virial EOS should only be used for vapors far from their critical point- pressures that are high enough to cause departures from ideal gas but low enough for Z to be in the “linear” region.
You might also like to view...
? Identify and state the historical significance of jeremiads.
What will be an ideal response?
Production by agricultural animals is never limited by age
Indicate whether the statement is true or false
Which type of motor can be easily reversed by switching two of the leads?
a. Squirrel cage motor b. Synchronous motor c. Three phase induction motor d. Brushless DC motor
Refer to the figure. Determine c-c length of pipe J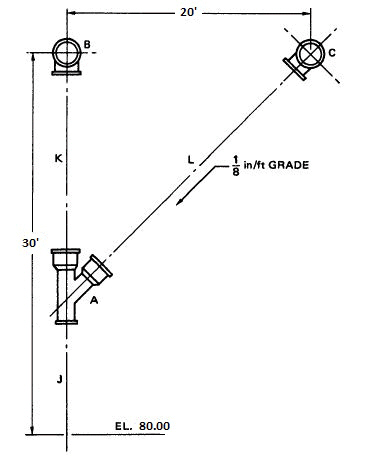
Fill in the blank(s) with the appropriate word(s).