The decomposition of nitrogen dioxide  into nitrogen monoxide
into nitrogen monoxide 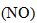 and oxygen is a second-order reaction. This means that the concentration C of
and oxygen is a second-order reaction. This means that the concentration C of 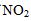 at time t is given by 1/C=kt+1/
at time t is given by 1/C=kt+1/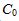 , where
, where 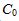 is the initial concentration and k is the rate constant. Assume the initial concentration is known to be 0.03 mol/L exactly. Assume that time can be measured with negligible uncertainty.
is the initial concentration and k is the rate constant. Assume the initial concentration is known to be 0.03 mol/L exactly. Assume that time can be measured with negligible uncertainty.
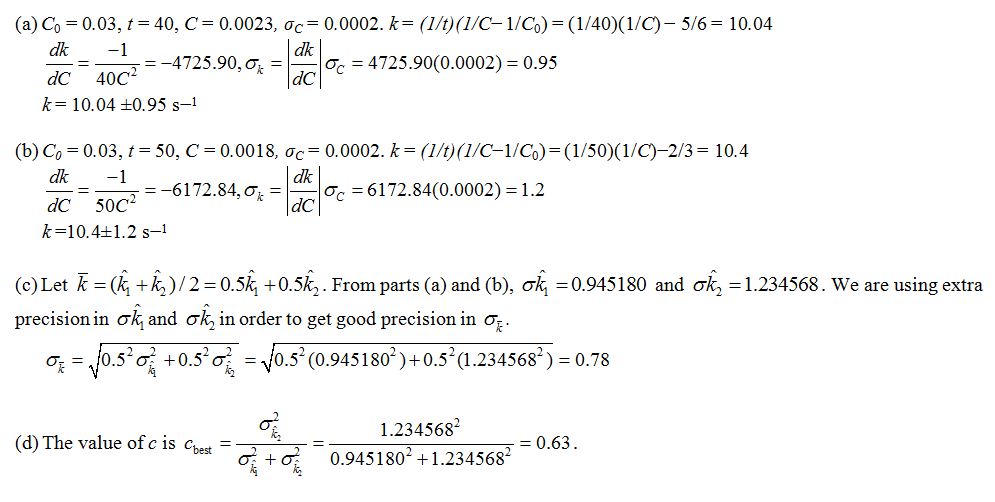
a. After 40 s, the concentration C is measured to be 0.0023 ± 2.0 ×10–4mol/L. Estimate the rate constant k, and find the uncertainty in the estimate.
b. After 50 s, the concentration C is measured to be 0.0018 ± 2.0 ×10–4mol/L. Estimate the rate constant k, and find the uncertainty in the estimate.
c. Denote the estimates of the rate constant k in parts (a) and (b) by 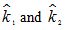 , respectively. The average
, respectively. The average 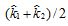 is used as an estimate of k. Find the uncertainty in this estimate.
is used as an estimate of k. Find the uncertainty in this estimate.
d. Find the value of c so that the weighted average 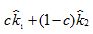 has the smallest uncertainty.
has the smallest uncertainty.
You might also like to view...
When measuring the resistance of a contactor coil, a reading of zero indicates _____.
a. that the coil is good b. an open coil c. a blown fuse d. a shorted coil
? Identify and state the historical significance of Adlai Stevenson.
What will be an ideal response?
What is the temperature rise of a furnace with a supply air temperature of 130°F and a return air temperature of 75°F, operating in an ambient temperature of 50°F?
A) 75°F - 50°F = 25°F temperature rise B) 130°F - 50°F = 80°F temperature rise C) 50°F + 75°F = 125°F temperature rise D) 130°F - 75°F = 55°F temperature rise
The linear feet of baseboard molding (not including waste) needed to trim a 9' × 12' room is _____
a. 21' b. 36' c. 42' d. 108'