An oxygen molecule falls in a vacuum. From what height must it fall so that its kinetic energy at the bottom equals the average energy of an oxygen molecule at 800 K?
(The Boltzmann constant is 1.38 × 10-23 J/K, the molecular weight of oxygen is 32.0 g/mol, and Avogadro's number is 6.022 × 1023 molecules/mol.)
A) 31.8 km
B) 10.6 km
C) 21.1 km
D) 42.3 km
Answer: A
You might also like to view...
An ideal gas is at a pressure of 2.00 atmospheres and a temperature of 35.0 What is the molar density?
What is the molar density?
A. 84.3 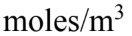
B. 78.9 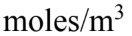
C. 70.2 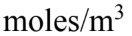
D. 66.7 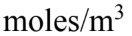
E. 60.4 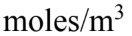
The decay constant of radon is 0.181 day s-1. A sample of radon contains 6.00 × 108 radon atoms. How many atoms are left after 10.0 days?
A) 9.82 × 107 B) 7.67 × 107 C) 8.34 × 108 D) 7.29 × 108 E) 8.56 × 108
A galvanometer calibrated to read current is
A) an ammeter. B) a voltmeter. C) an ohm meter. D) none of the above
A bicycle whose wheels have a radius of 66 cm is traveling at 2.0 m/s. If the wheels do not slip, what is the angular speed of the wheels?
What will be an ideal response?