First Law of Thermodynamics: A monatomic ideal gas undergoes an isothermal expansion at 300 K, as the volume increased from 0.010 m3 to 0.040 m3. The final pressure is 130 kPa. What is the change in the internal (thermal) energy of the gas during this process? (R = 8.31 J/mol ? K)
A. 0.0 kJ
B. 3.6 kJ
C. 7.2 kJ
D. -3.6 kJ
E. -7.2 kJ
Answer: A
You might also like to view...
General Questions: The figure shows two boxes, with m1 > m2, that are on a level frictionless surface. We can apply a horizontal force 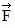 either toward the right on m1 or toward the left on m2. The magnitude of the force that the boxes exert on each other is
either toward the right on m1 or toward the left on m2. The magnitude of the force that the boxes exert on each other is
A. zero newtons in either case.
B. larger if  is applied toward the right.
is applied toward the right.
C. larger if  is applied toward the left.
is applied toward the left.
D. the same in either case.
A horizontal tube consists of a 7.0-cm diameter pipe that narrows to a 2.0-cm-diameter throat. In the pipe, the water pressure is twice atmospheric pressure and the water flows with a speed of 0.40 m/s
What is the pressure in the throat, assuming that the water behaves like an ideal fluid? The density of water is 1000 kg/m3, and atmospheric pressure is 1.01 × 105 Pa. A) 1.9 atm B) 0.12 atm C) 2.1 atm D) 2.0 atm
A solid object has a volume density ?0 at a temperature of 315 K. The coefficient of volume expansion for the material of which it is made is 7.00 × 10-5 K-1
What will be its density (in terms of ?0 at a temperature of 425 K, assuming that it does not melt and that its thermal properties do not change with temperature?
What is the correct electronic configuration for the ground state sodium atom, which has 11 electrons?
A) 1s12s23p62s2 B) 1s22s22p63s2 C) 1s12s22p6 D) 1s22s13p62s2 E) 1s22s22p63s1