Which of the following statements about the atomic theory of matter is true?
Called the "father of atomic theory," Dalton's theories of molecules and different elements in proportion led the charge towards accepting the concept of the atom.
You might also like to view...
The magnitude of the component of the force that does the work is 43.0 N. How much work is done on a bookshelf being pulled 5.00 m at an angle of 37.0 from the horizontal?
a. 172 J b. 215 J c. 129 J d. 792 J
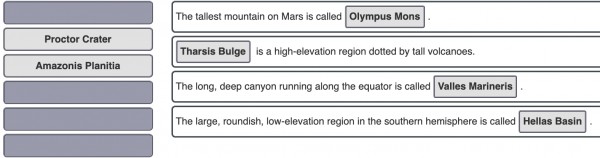
Now go to the screen "Major Geological Features," and explore the main screen with labels on and off and elevation on and off. Then complete the sentences below.
A parallel plate capacitor is charged to 12 V and the voltage source is then disconnected. When the air space between the plates is then filled with a dielectric of constant 6.0, what is the resulting voltage between the plates?
A. 72 V B. 12 V C. 6.0 V D. 2.0 V E. none of these choices are correct
2.00 moles of an ideal gas is at a pressure of 1.50 atmospheres and a volume of 0.00100 m3. What is the total kinetic energy of the gas?
A. 175 J B. 228 J C. 267 J D. 303 J E. 367 J