A conductive heat transfer of 15 W is applied to a metal bar whose length is 0.50- m. The hot end of the bar is at 80oC. Determine the temperature at the other end of the bar for (a) a copper bar (? = 401 W/m-K) with a cross-sectional area of 0.0005 m2, (b) a copper bar (? = 401 W/m-K) with a cross-sectional area of 0.005 m2, and (c) a zinc bar (? = 116 W/m-K) with a cross-sectional area of 0.005 m2.
Given: Q?cond=150 W; ?x = 0.50 m; T1 = 80°C
What will be an ideal response?
From Qcond = ?A dT/dx = - ?A T2-T1/?x
T2 = Qcond/-?A + T1
(a) Copper, ? = 401 W/m-K, A = 0.0005 m2
T2 = 15W/-(401W/m-K) (0.0005 m2) (0.50 m) + 80° = 42.6°C
(b) Copper, ? = 401 W/m-K, A = 0.005 m2
T2 = 76.3°C
(c) Zinc, ? = 116 W/m-K, A = 0.005 m2
T2 = 54.1°C
You might also like to view...
Rule #1 is referred to as the “____________________.”
Fill in the blank(s) with the appropriate word(s).
Bad ____ are a common cause of AC generator charging problems.
A. diodes B. ohmmeters C. ferrules D. rotors
Radiocarbon dating: Carbon-14 is a radioactive isotope of carbon that decays by emitting a beta particle. In the earth’s atmosphere, approximately one carbon atom in 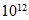 is carbon-14. Living organisms exchange carbon with the atmosphere, so this same ratio holds for living tissue. After an organism dies, it stops exchanging carbon with its environment, and its carbon-14 ratio decreases exponentially with time. The rate at which beta particles are emitted from a given mass of carbon is proportional to the carbon-14 ratio, so this rate decreases exponentially with time as well. By measuring the rate of beta emissions in a sample of tissue, the time since the death of the organism can be estimated. Specifically, it
is carbon-14. Living organisms exchange carbon with the atmosphere, so this same ratio holds for living tissue. After an organism dies, it stops exchanging carbon with its environment, and its carbon-14 ratio decreases exponentially with time. The rate at which beta particles are emitted from a given mass of carbon is proportional to the carbon-14 ratio, so this rate decreases exponentially with time as well. By measuring the rate of beta emissions in a sample of tissue, the time since the death of the organism can be estimated. Specifically, it
is known that t years after death, the number of beta particle emissions occurring in any given time interval from 1 g of carbon follows a Poisson distribution with rate 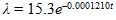 events per minute. The number of years t since the death of an organism can therefore be expressed in terms of ?:
events per minute. The number of years t since the death of an organism can therefore be expressed in terms of ?:
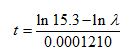
An archaeologist finds a small piece of charcoal from an ancient campsite. The charcoal contains 1 g of carbon.
a. Unknown to the archaeologist, the charcoal is 11,000 years old. What is the true value of the emission rate ??
b. The archaeologist plans to count the number X of emissions in a 25 minute interval. Find the mean and standard deviation of X.
c. The archaeologist then plans to estimate ? with 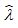 = X/25 . What is the mean and standard deviation of
= X/25 . What is the mean and standard deviation of 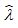 ?
?
d. What value for 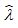 would result in an age estimate of 10,000 years?
would result in an age estimate of 10,000 years?
e. What value for 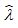 would result in an age estimate of 12,000 years?
would result in an age estimate of 12,000 years?
f. What is the probability that the age estimate is correct to within ±1000 years?
What additive in oil aides in the prevention of varnish buildup?
A. antioxidation agents B. viscosity index improver C. antifoaming agents D. cohesion agents