If you bubble pure H2S gas into a beaker of water, what is the concentration of HS- if the pH is controlled and remains at 5.
The Henry’s Law constant for 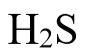 is 0.1 mole/L-atm, and
is 0.1 mole/L-atm, and 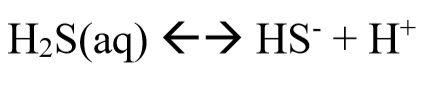
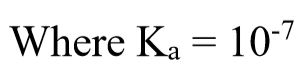
a. moles/L
b. mg/L
What will be an ideal response?
a. moles/L
Step 1: Use Henry’s Law to find the total dissolved sulfur species pure gas.
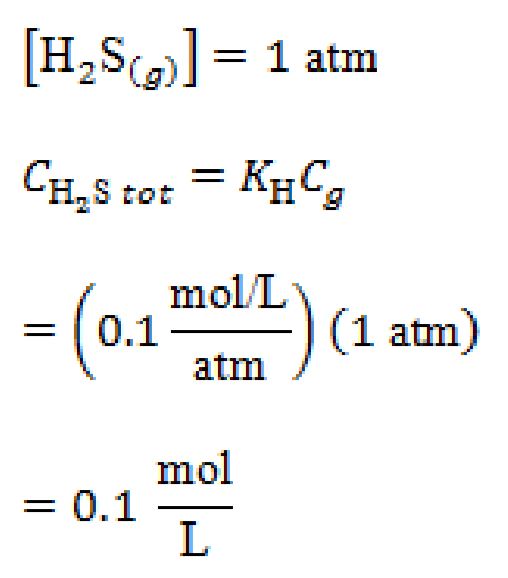
Step 2: Use equilibrium constant
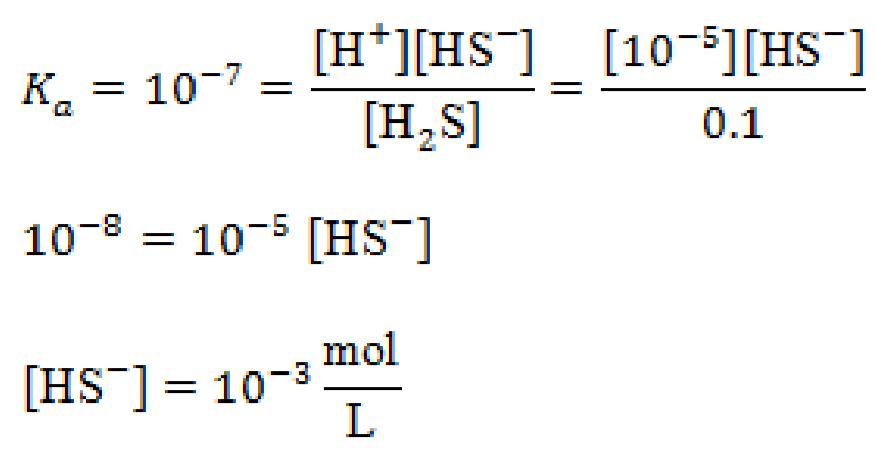
b. mg/L, and
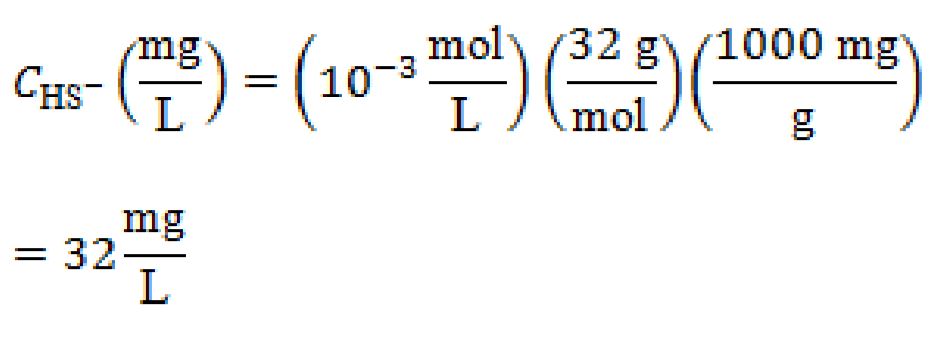
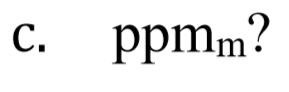
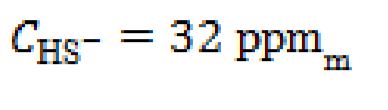
You might also like to view...
The pH scale measures the ____________________ or ____________________ of a substance
Fill in the blank(s) with correct word
The cruise control system is inoperative. Technician A says a faulty brake light switch can be the cause. Technician B says the cause may be the dump valve is stuck open. Who is correct?
A. Technician A only B. Technician B only C. Both Technician A and B D. Neither Technician A nor B
The purpose of purging the lines with nitrogen during brazing is to:
A) Pressurize the lines for leak testing. B) Reduce the amount of refrigerant that must be added once the installation is completed. C) Prevent the formation of copper oxide inside the lines. D) Increase the temperature of the torch flame and decrease brazing time.
For fastening two pieces of rough lumber together, the nail used would be a ____.
A. finishing nail B. common nail C. roofing nail D. masonry nail