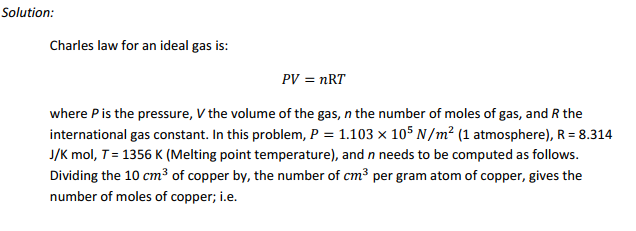
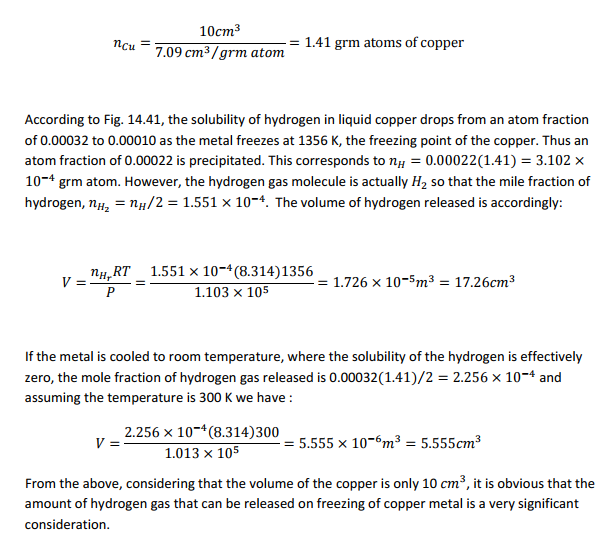
Care must be taken in melting certain copper alloys to prevent absorption of hydrogen from the furnace gases or as a result of reaction with water vapor. The release of this gas on freezing can result in an open porous structure of little value. As an exercise, compute the volume of hydrogen that should be released in 10 cc of copper melted in a hydrogen atmosphere at a pressure of one atmosphere when the metal is frozen. The atomic volume of copper is 7.09 ??? per gm atom. Assume the ideal gas law and refer to Fig. 14.41.
What will be an ideal response?
You might also like to view...
Identify the following factors as either tangible or intangible: sustainability, installation cost, transportation cost, simplicity, taxes, resale value, morale, rate of return, dependability, inflation, acceptance by others, and ethics.
What will be an ideal response?
The responsibilities of an animal scientist working for an agricultural organization commonly include:
a. educating the public about the products and production systems b. serving farmer members c. working with legislators to protect the interests of agriculture d. all answers
The _____ prevents the engine starting unless the clutch pedal is fully depressed.
A. clutch safety switch B. reverse lamp switch C. upshift lamp circuit D. electrical clutch
GMAW power supplies are:
A) DCEN B) Constant current C) Constant voltage D) AC/DC