By inspection of the figure, which of the following statements is false?
Consider the following figure that describes the solid-liquid equilibrium of a binary mixture:
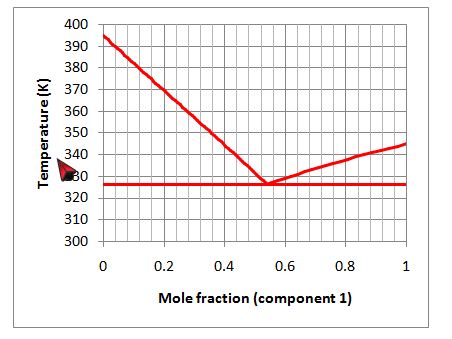
A. The melting point of component 1 is about 345 K.
B. The system would be reasonably modeled by an ideal solution for the liquid and an ideal solution for the solid.
C. The system forms an immiscible solid solution.
D. The melting point of component 2 is about 395 K.
E. None of the above are false.
A. Incorrect. This is true. Setting the mole fraction to 1 we obtain the melting point of component 1, which is around 345 K
B. Correct. This is false. The fact that the liquid has a eutectic means that it will not be reasonably modeled by an ideal solid solution.
C. Incorrect. This is true. We see no regions of single solid phase at the high concentrations of either component. Below the solidus line, two phases of pure component 1 and pure component 2 will form.
D. Incorrect. This is true. Setting the mole fraction to 0, we find that this is the melting point of pure component 2, which is around 395 K.
E. Incorrect. One statement is false.
You might also like to view...
Subtract 56°19' from 73°34'.
Fill in the blank(s) with the appropriate word(s).
What plant activities can be regulated by plant hormones?
What will be an ideal response?
What is the decimal value of the 2's-complement signed binary number 110101101?
A) -8310 B) +l7410 C) +8310 D) -17410
The term ____ refers to the railing that you slide your hand along as you walk down the stairs.
A. headroom B. handrail C. kicker D. guardrail