One mole of an ideal gas with 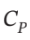 = (5/2)R and
= (5/2)R and 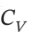 = (3/2)R expands from
= (3/2)R expands from 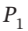 = 6 bar and
= 6 bar and 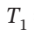 = 800 K to
= 800 K to 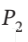 = 1 bar by each of the following paths:
= 1 bar by each of the following paths:
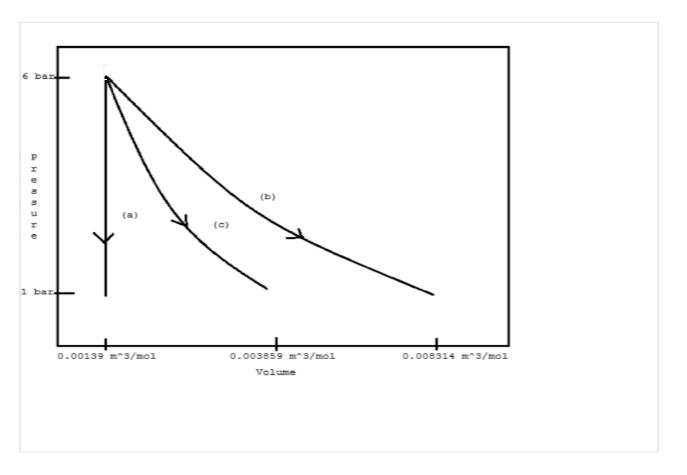
(a) Constant volume
(b) Constant temperature
(c) Adiabatically
Assuming mechanical reversibility, calculate W, Q, ?U, and ?H for each process. Sketch each path on a single PV diagram.
For each of these problems start with equations
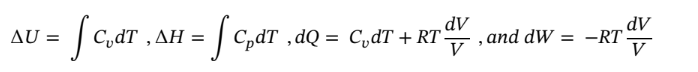
a. at constant volume,
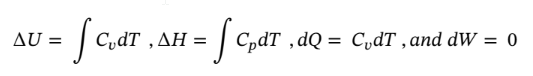
So plugging the given values into each part gives:
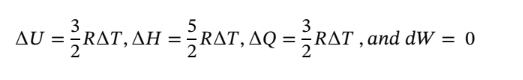
Now not knowing what 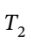 is, we must assume to stay at an ideal gas that since the pressure drops by a factor of 6, the temperature must drop by the same as well, leaving
is, we must assume to stay at an ideal gas that since the pressure drops by a factor of 6, the temperature must drop by the same as well, leaving 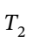 = 800/6 = 133.33 K. Using this value gives the following values:
= 800/6 = 133.33 K. Using this value gives the following values:
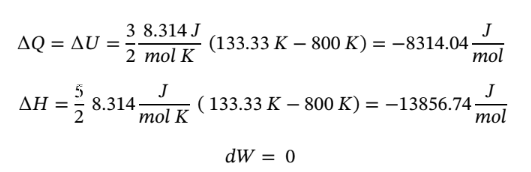
b. Using the same starting point as in part (a) and assuming constant temperature, reduces the equations to the following:
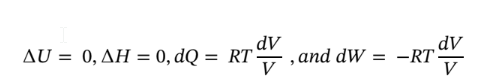
Putting dQ and dW in terms of pressure and integrating gives:
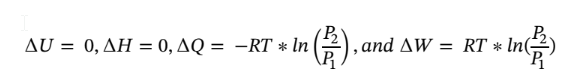
Putting in the known values and solving gives
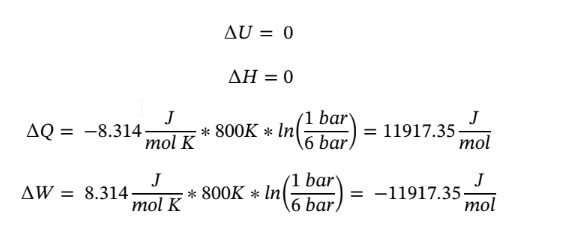
c. Again starting with the same equations as in part (a) and assume dQ = 0 (adiabatic) gives:
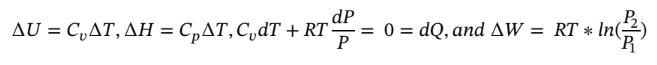
However, we also have adiabatic expansion which changes  integrating and solving for
integrating and solving for 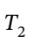 gives:
gives:
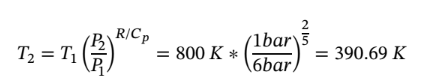
Plugging in given values and solving gives:
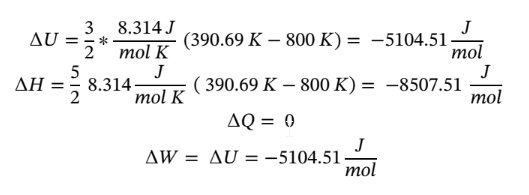
You might also like to view...
Minimum breaking strength for lanyards used in personal fall protection systems must be _____.
a. 2,000 pounds b. 3,000 pounds c. 4,000 pounds d. 5,000 pounds
What is the likely performance outcome of a defective coil pack in a CPS SI system used in a current CNG engine??
A. Misfire B. ?Increased fuel consumption C. ?Dead cylinder D. ?All of the above
A CAD drafter locates circles with diameters of 1.575 mm, 1.625 mm, 3.850 mm, 3.583 mm, 4.45 mm, 1.95 mm, and 5.05 mm on a drawing. Find, to the nearest thousandth, the average length of these diameters.
What will be an ideal response?
Only ________ is ahead of the United States in annual hog production.
A. France B. Italy C. China D. Denmark