Solve.The pressure of a gas varies jointly as the amount of the gas (measured in moles) and the temperature and inversely as the volume of the gas. If the pressure is 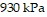 (kiloPascals) when the number of moles is 4, the temperature is
(kiloPascals) when the number of moles is 4, the temperature is 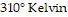 , and the volume is
, and the volume is 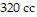 , find the pressure when the number of moles is 6, the temperature is
, find the pressure when the number of moles is 6, the temperature is  , and the volume is
, and the volume is
src="https://sciemce.com/media/4/ppg__10619191748__f1q49g5.jpg" alt="" style="vertical-align: -4.0px;" />.
A. 1400
B. 680
C. 660
D. 1360
Answer: B
Mathematics
src="https://sciemce.com/media/4/ppg__10619191748__f1q49g5.jpg" alt="" style="vertical-align: -4.0px;" />.
A. 1400
B. 680
C. 660
D. 1360
Answer: B
Mathematics
You might also like to view...
Solve.m - 4 ? -2
A. m ? 6 B. m ? 2 C. m ? -2 D. m ? -6
Mathematics
Write the algebraic expression without parentheses.-5(5z)
A. -25 - 5z B. -25 + z C. 25z D. -25z
Mathematics
Evaluate the expression without using a calculator.84/3
A. 128 B. 32 C. 64 D. 16
Mathematics
Write an equation of the line passing through the given points.(9, -55) and (-7, 25)
A. y = 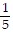 x -
x - 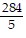
B. y = -5x - 10
C. y = 5x - 100
D. y = - 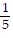 x -
x - 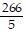
Mathematics