A 200-ml solution of 0.5 M lactic acid was adjusted to a pH of 3.6 . If you add 20 ml of 2.0 M NaOH what will the pH of the solution be? The pKa of lactic acid is 3.6
A. 4.6
B. 3.6
C. 3.0
D. 2.6
E. 2.0
A
You might also like to view...
In the experiment above, guppy color patterns (spots) were measured in populations exposed to increasing amounts of predation. From this you could conclude that ____ .
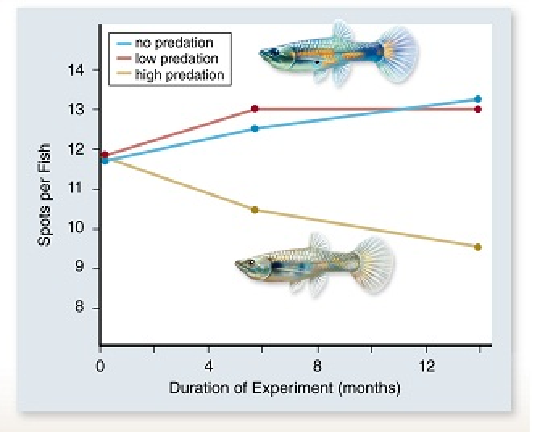
A. evolutionary changes take millions of years to appear
B. predators are less likely to catch and eat brightly colored guppies
C. predators are more likely to catch and eat brightly colored guppies
D. brightly colored guppies are more likely to reproduce in the presence of predators
E. predators do not affect the color patterns of guppies
The endospores of Pseudomonas make that organism very difficult to kill
Indicate whether this statement is true or false.
How does the binding of a regulatory molecule to the allosteric site affect the activity of an enzyme? (Check all that apply.)
A. It may change the maximum velocity of the enzyme. B. It may decrease the activity of the enzyme. C. It may increase the activity of the enzyme. D. It may change the affinity of the enzyme for its substrate. E. It causes the enzyme to denature and become inactive. F. It may cause the active site to disappear altogether. G. It may change the shape of the enzyme.
Genes that have been altered and may cause cancer are called
1.proto-oncogenes. 2.oncogenes. 3.totipotent genes. 4.controlled genes. 5.constitutive genes.