Solve the problem.The pH of a solution ranges from 0 to 14. An acid has a pH less than 7. Pure water is neutral and has a pH of 7. The pH of a solution is given by 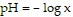 where x represents the concentration of the hydrogen ions in the solution in moles per liter. Find the pH if the hydrogen ion concentration is
where x represents the concentration of the hydrogen ions in the solution in moles per liter. Find the pH if the hydrogen ion concentration is 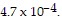
A. 4.33
B. 4.67
C. 3.33
D. 3.67
Answer: C
Mathematics
You might also like to view...
Solve.A fish tank holds 6.0 liters of water. How many gallons does the tank hold, to the nearest tenth?
A. 6.4 gal B. 1.6 gal C. 5.7 gal D. 22.7 gal
Mathematics
Perform the operations. 
A. 19 B. 16 C. 144 D. 17
Mathematics
Simplify.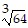
A. 4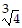
B. 16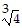
C. 4
D. 4 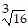
Mathematics
Write the set in set-builder notation.{a, b, c, . . . , x, y, z}
A. {x|x is a vowel} B. {x|x is a letter of the alphabet} C. {x|x is a consonant} D. {x|x is a number}
Mathematics