A 360-g metal container, insulated on the outside, holds 180.0 g of water in thermal equilibrium at 22.0°C. A 24.0-g ice cube, at the melting point, is dropped into the water, and when thermal equilibrium is reached the temperature is 15.0°C
Assume there is no heat exchange with the surroundings. For water, the specific heat capacity is 4190 J/kg ? K and the heat of fusion is 3.34 × 105 J/kg. What is the specific heat capacity of the metal of the container? A) 1700 J/kg ? K
B) 970 J/kg ? K
C) 2300 J/kg ? K
D) 2800 J/kg ? K
E) 3300 J/kg ? K
A
You might also like to view...
Briefly describe Mars's surface
What will be an ideal response?
When the distance between two charges is halved, the electric force between the charges
A) quadruples. B) doubles. C) is half. D) is reduced by one-quarter. E) none of the above
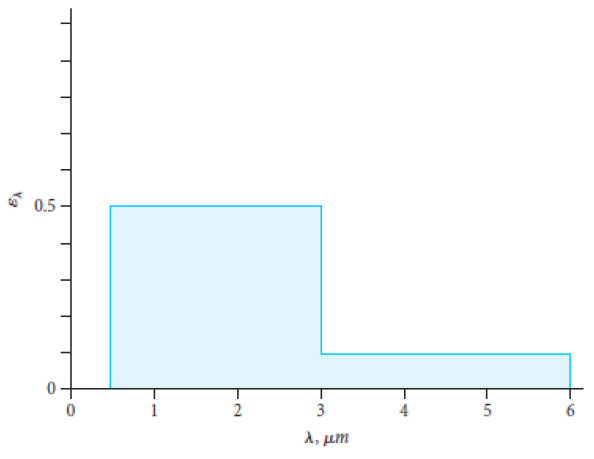
For the gray opaque surface having the spectral emissivity given in the figure, determine the spectral reflectivity.
Astronomers can use ________ to measure magnetic fields on the Sun
a. helioseismology b. perchloroethylene (C2Cl4) c. neutrino detectors d. a magnetic carpet e. the Zeeman effect