Which of the following statements is untrue about oxidation and reduction processes?
A. Electrons are often involved in oxidation and reduction reactions.
B. An oxidation can happen without a reduction.
C. Often ions are generated or consumed.
D. It involves the exchange of electrons.
E. All of the above are true.
Answer: B
You might also like to view...
Which of the following locations would form an igneous rock?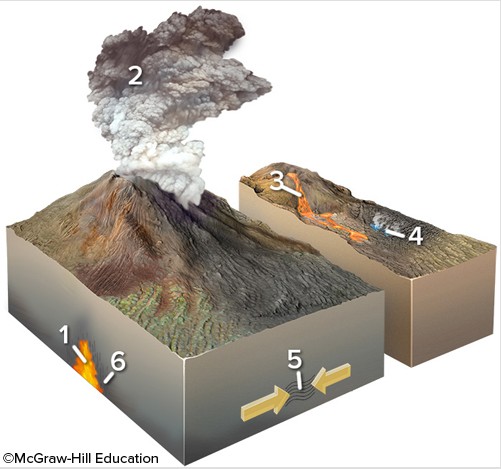
A. locations 3 and 4 B. locations 1 and 2 C. locations 2 and 3 D. locations 5 and 6 E. locations 1, 2, and 3
Which bond would you expect to find in halite (NaCl)?
a. valence electron bonds b. covalent bonds c. metallic bonds d. hybrid bonds e. ionic bonds
The "four Rs" of waste reduction present an alternative to the ____ economy.
A. sustainable B. ecosystem C. recycling D. profitable E. throwaway
All of the following countries of Asia are described as new emerging "tigers," EXCEPT
A) South Korea. B) Thailand. C) Malaysia. D) Indonesia. E) China.