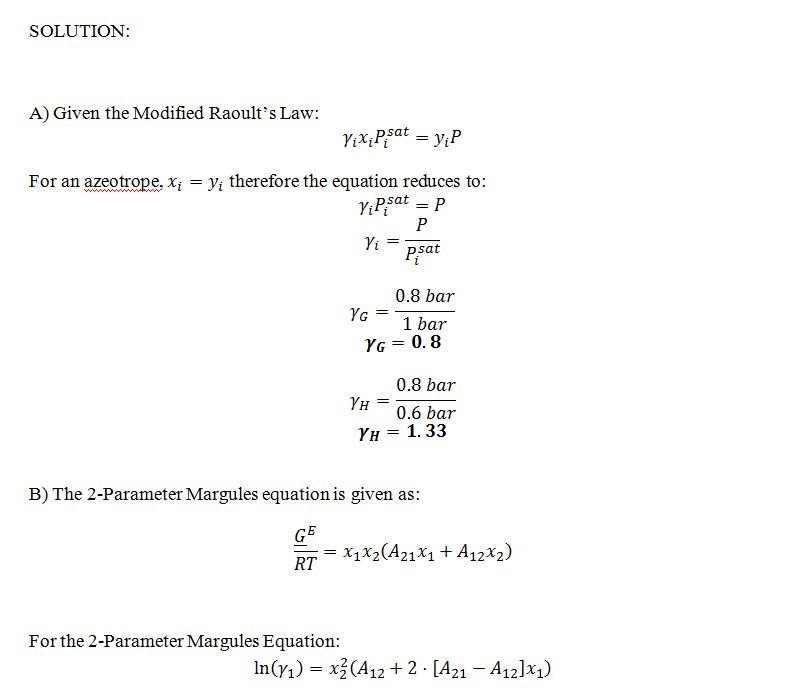
Compounds G and H form non-ideal liquid solutions that can be described by the two-parameter Margules equation. The following is known:
• The vapor pressure of pure compound G at T=300 K is 1 bar.
• The vapor pressure of pure compound H at T=300 K is 0.6 bar.
• At T=300 K and P=0.8 bar, there is an azeotrope at which liquid and vapor are in equilibrium and both phases have a composition of 70% G and 30% H.
A) Find the activity coefficients of compounds G and H in the azeotropic mixture described above.
B) Find a general expression for the excess Gibbs energy of a liquid mixture of G and H at T=300 K. The only unknowns in your expression should be the mole fractions of G and H in the liquid phase.
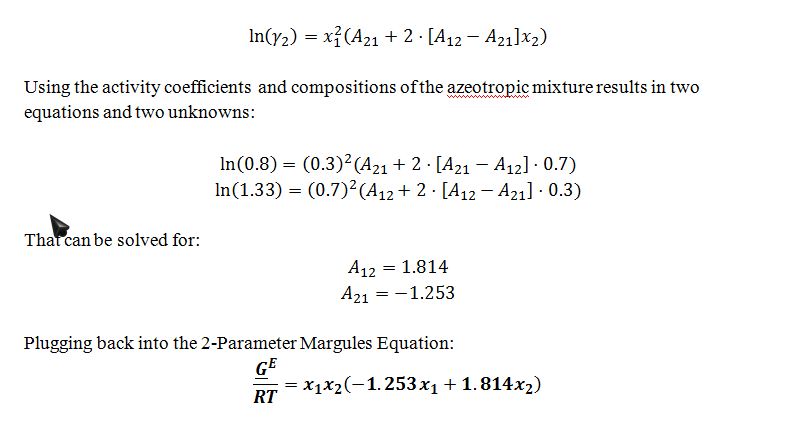
You might also like to view...
An entrepreneur proposes to generate 34 hp by using 20 kg/min of hot water (99ºC) from a hot spring and exhausting it to a 15ºC stream. Select the best conclusion after analyzing the proposal:
A) It’s impossible B) It should be pursued C) It’s improbable D) Additional information is required QH = 900 kJ/sEngine
The amount of different sizes of mineral particles in a soil defines the soil ________
A) solution B) texture C) pore space D) structure E) profile
Why is it important to make the braze on a compressor stub as quickly as possible?
What will be an ideal response?
Recharging the battery pack in a BEV normally takes several ____.
A. seconds B. minutes C. hours D. days