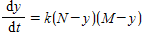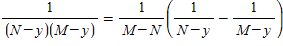Two chemical solutions, one containing N molecules of chemical A and another containing M molecules of chemical B, are mixed together at time t = 0. The molecules from the two chemicals combine to form another chemical solution containing y (AB) molecules. The rate at which the AB molecules are formed, 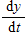 , is called the reaction rate and is jointly proportional to
, is called the reaction rate and is jointly proportional to ![]()
src="https://sciemce.com/media/3/ppg__cognero__9.3_Applications_of_Separable_Differential_Equations__media__920f4707-5d77-44d6-867e-3d7dea6ed07c.PNG" style="vertical-align: middle;" /> and 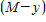
?
?
where k is a constant (we assume the temperature of the chemical mixture remains constant during the interaction). Solve this differential equation with the side condition 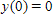
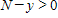
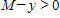
?
Hint: Use the identity
?
What will be an ideal response?
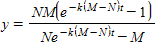
You might also like to view...
Solve the problem.A carpeted living room and dining room area measures 36 ft by 10 ft. Mark decides to install wood flooring in the 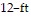 by
by 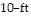 dining room. By what percent has he reduced the area that is carpeted?
dining room. By what percent has he reduced the area that is carpeted?
A. 10% B. 66.7% C. 33.3% D. 50%
Provide an appropriate response.Explain why 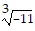 and -
and -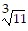 represent the same number.
represent the same number.
What will be an ideal response?
Divide.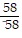
A. 1
B. 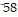
C. 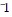
D. 0
Find anand a6 for the following arithmetic sequence.a1 = -50, d = -3
A. an = -50 + 3n, a6 = -32 B. an = -50 - 3n, a6 = -68 C. an = -50 - 3(n - 1), a6 = -65 D. an = -50 + 3(n - 1), a6 = -35