Which assumption that you made in solving this problem would you characterize as least valid and why?
A liquid mixture of diethyl ketone (1) and n-hexane (2) is fed into a flash distiller operating at 328 K and 0.45 bar. The feed rate is 2.15 mol/s and the feed is 40% (by mol) of the n-hexane. What is the composition and amount of the resulting phase(s)? Note that the vapor pressure of diethyl ketone at this temperature is 0.29 bar and that of the n-hexane is 0.9015 bar. Answer the question using ONLY the given information.
Overall Balance:
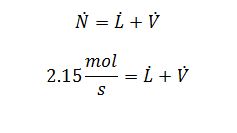
Component Balance on (1):
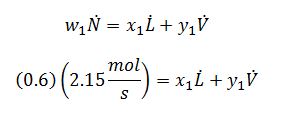
Raoult’s Law on (1):
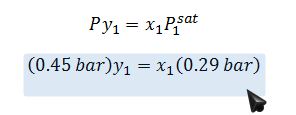
Raoult’s Law on (2):
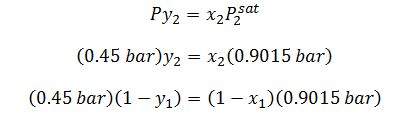
Plug set of 4 equations into solution software to solve for the 4 unknowns:
L ?=1.018 mol/s
V ?=1.132 mol/s
x_1=0.738
y_1=0.476
The least valid assumption made was assuming that the liquid solution was ideal. A mixture of diethyl ketone which is polar and n-hexane which is non-polar is usually not treated as an ideal solution due to the non-polar to polar interactions that exist. But a better answer that uses a real solution model is not possible to implement from just the given information.
?
You might also like to view...
____________________ generating equipment for home installation is now available from several manufacturers and can be installed by a professional service or by the homeowner.
Fill in the blank(s) with the appropriate word(s).
The higher area in the middle of an athletic field is called the ____________________
Fill in the blank(s) with correct word
What is the difference between Grade A and Grade B milk?
What will be an ideal response?
What device on the engine is responsible for manifold-boost?
A. Piston B. Turbocharger C. Exhaust gas recirculation system D. Manifold