State the specific heat of water in terms of standard SI energy units (joules)
In the cgs system of units, specific heat is the amount of heat required to raise one
gram of a substance one degree Celsius. Heat capacity is the amount of heat required
to raise the temperature of the given mass (amount varies) one degree Celsius. Heat
capacity = mass × specific heat.
You might also like to view...
Series/Parallel Circuits: For the circuit shown in the figure, R1 = 5.6 ?, R2 = 5.6 ?, R3 = 14 ?, and ? = 6.0 V, and the battery is ideal.(a) What is the equivalent resistance across the battery?(b) Find the current through each resistor.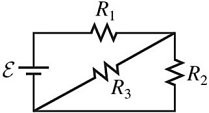
What will be an ideal response?
Figure Q6.15 below shows a sequence of Stern–Gerlach devices. By analogy to the cases discussed in the chapter, what do you think are the probabilities that an electron entering the last device will come out of that device’s plus and minus channels, respectively? 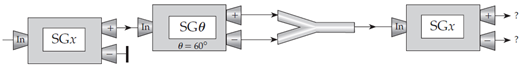
A. 1 and 0, respectively (AA if reversed, i.e., 0 and 1)
B. 0.933 and 0.067, respectively (BB if reversed)
C.  , respectively (CC if reversed)
, respectively (CC if reversed)
D. 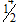 for both channels
for both channels
E. Some other probabilities (specify)
A 1.20-kg ball is hanging from the end of a rope. The rope hangs at an angle 25.0° from the vertical when a 15.0 m/s horizontal wind is blowing. If the wind's force on the rope is negligible, what drag force does the wind exert on the ball?
A) 32.3 N B) 24.1 N C) 3.68 N D) 5.48 N E) 11.8 N
What is an inverse-square relationship between force and distance?
a. A mathematical expression for the distance in terms of force instead of force in terms of distance, which is then squared. b. A relationship where increasing the distance by a factor increases the force by the square of the same factor. c. A relationship where one value decreases in proportion to the square of the factor by which the other value decreases. d. A relationship where the increasing the distance by a factor decreases the force by dividing it by the square of that same factor. e. A relationship where the square of force is proportional to the square of the distance.